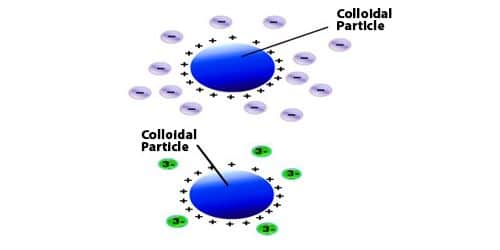
Hardy-Schulze rule: The coagulation power of precipitated ion is more if its valency is high. For example, coagulation power series of Al3+, Na+ and Ba2+ is Al3+ > Ba2+ > Na+.
In a sentence this rule state as “Greater is the valency of the oppositely charged ion of the electrolyte being added, the faster is the coagulation”.
Hardy Schulze law:-
- The efficient ions of the electrolyte in bringing about coagulation are those which hold charge reverse to that of the colloidal particles. These ions are called coagulating ions.
- Greater is the valency of the coagulating or the Flocculating ion, greater is its power to bring about the coagulation.
- Power to cause precipitation increases: Al3+ > Ba2+ > Na+
The dissimilar electrolytes have diverse coagulation capacity which depends upon the valency of the active ion, known as a flocculating ion (carrying a charge opposite to the charge of the colloidal particles).
Explanation
Coagulating ions are the ions dependable for causing coagulation of colloidal solution. Ions are unsteady and bigger their valency, larger is the ionic volatility. Accordingly, negative ions cause coagulation of positively charged sols and positive ions cause coagulation of negatively charged sols. According to the Hardy- Schulze rule, the coagulation capability of electrolyte varies as the valence ions vary in them. So when the valency of the active ion will be greater the coagulating power of the ion will also be greater.
Generally, a coagulation procedure occurs by the accumulation of electrolytes. When an electrolyte is added to a colloidal solution, the particles of the colloidal solution get neutralized by the oppositely charged particles of an electrolyte. Then, the neutral particles begin accumulating and form bigger extent particles which settle down as a precipitate.
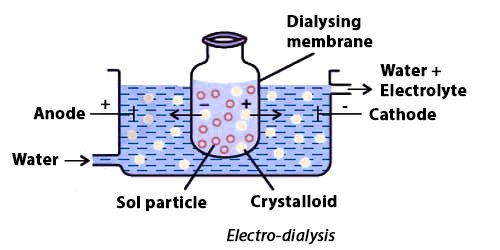
Fig: Purification of colloidal solutions
According to Hardy-Schulze rule, greater the valency of the active ion or flocculating ion, greater will be its coagulating power. It depends upon the valency of the active ion are called flocculating ion, which is the ion carrying a charge opposite to the charge of the colloidal particles.
Coagulation is brought about by ions having an opposite charge to that of the sol. Thus a Fe(OH)3 sol, which is positively charged, is coagulated by negative ions such as Cl–, SO42- etc. Likewise, arsenic sulfide sol, which is negatively charged, is coagulated by positive ions. Thus greater the valency of active ion greater is the coagulating power of that iron.
Limitation
This law takes into contemplation only the charge carried by an ion, not its size. The lesser the size of an ion, the more will be its polarizing control. Thus, Hardy-Schulze law can be modified in terms of the polarizing power of the flocculating ion. Thus, the modified Hardy-Schulze law can be stated as ‘the greater the polarizing power of the flocculating ion added, the greater is its power to cause precipitation.’