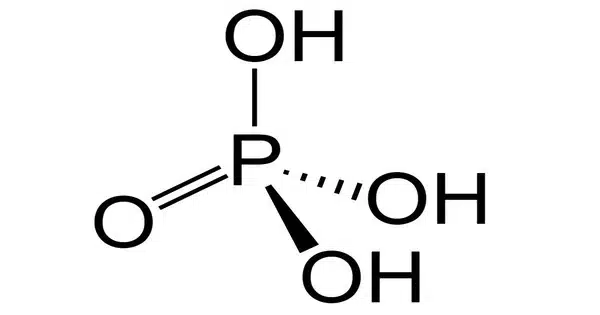
Phosphoric acid is a colorless, odorless, inorganic phosphorus-containing substance having the chemical formula H3PO4. It is most usually encountered in the form of an 85% aqueous solution, which is a colorless, odorless, non-volatile syrupy liquid. It is a mineral acid that has several industrial and chemical applications. It is a significant industrial chemical that is found in many fertilizers.
In comparison to powerful mineral acids such as sulfuric acid or hydrochloric acid, phosphoric acid is a weak acid. It has a lower dissociation constant (pKa) than stronger acids, making it less acidic.
The name “orthophosphoric acid” can be used to distinguish this specific acid from other “phosphoric acids”, such as pyrophosphoric acid. Nevertheless, the term “phosphoric acid” often means this specific compound; and that is the current IUPAC nomenclature.
Properties
- Chemical formula: H3PO4
- Molar mass: 97.994 g·mol−1
- Appearance: Colorless solid
- Odor: Odorless
- Density: 1.6845 g/cm3 (25 °C, 85%), 1.834 g/cm3 (solid)
- Melting point: 42.35 °C (108.23 °F; 315.50 K) anhydrous 29.32 °C (84.78 °F; 302.47 K) hemihydrate
- Boiling point: 212 °C (414 °F) (only water evaporates)
- Solubility in water: 392.2 g/(100 g) (−16.3 °C); 548 g/(100 mL) (20 °C)[6]
- Solubility: Soluble in ethanol
Chemical Structure
Phosphoric acid is a triprotic acid, meaning it can donate three protons (H+ ions) in successive reactions. It consists of three hydrogen atoms, one phosphorus atom, and four oxygen atoms.
Production
Phosphoric acid is commonly produced by treating phosphate rock with sulfuric acid. The resulting product is typically a mixture of phosphoric acid, water, and various impurities. This mixture is often further processed to obtain more concentrated forms of phosphoric acid.
Uses
Phosphoric acid is often used in soft drinks and other processed foods as an acidulant and flavoring agent. It has a sour or acidic flavor. It is used in the making of naval jelly and as a rust converter to eliminate rust and corrosion from metals. It is also used in the creation of fertilizers such as ammonium phosphate and superphosphate fertilizers, which deliver necessary phosphorus to plants.
It can be used to make certain medications and in acid-base titrations. It’s found in dental products including dental cement and etching solutions.
Safety
The skin, eyes, and mucous membranes can all be harmed by phosphoric acid. When working with this chemical, certain safety procedures, such as donning protective clothing and handling it with care, are required.
There are several health problems associated with excessive phosphoric acid ingestion in the form of soft drinks. Due to its effect on calcium absorption, excessive consumption may contribute to teeth erosion and bone health concerns.