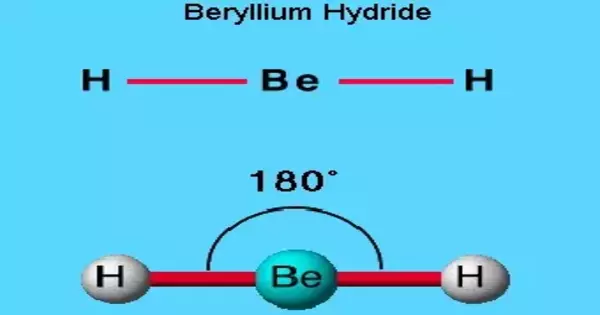
Beryllium hydride is a chemical compound with the formula (BeH2)n. It is a simple light metal hydride that is well known for its ability to store hydrogen. This alkaline earth hydride is a colorless solid that is insoluble in non-decomposing solvents. Beryllium hydride is covalently bonded, as opposed to the ionically bonded hydrides of the heavier Group 2 elements (three-center two-electron bond). It has no common applications. All beryllium compounds are extremely dangerous.
Beryllium hydride is an example of a covalent hydride. Because of the large electronegativity difference between Be and H. Crystalline BeH2 has been described as a three-dimensional array of tetrahedral BeH4 molecules rather than as BeH2 chains.
Properties
Beryllium Hydride, BeH2 is an amorphous, colorless, highly toxic polymeric solid (H = 18.3%) that is stable to water but hydrolyzed by acid. It is insoluble in organic solvents but reacts with tertiary amines at 160 °C to form stable adducts. It was formerly of interest as a rocket fuel and as a moderator for nuclear reactors.
- Chemical formula: BeH2
- Molar mass: 11.03 g mol−1
- Appearance: white solid
- Density: 0.65 g/cm3
- Melting point: 250 °C (482 °F; 523 K) decomposes
- Solubility in water: decomposes
- Solubility: insoluble in diethyl ether, toluene
Synthesis
Unlike the other group 2 metals, beryllium does not react with hydrogen. Instead, BeH2 is prepared from preformed beryllium(II) compounds. It was first synthesised in 1951 by treating dimethylberyllium, Be(CH3)2, with lithium aluminium hydride, LiAlH4.
Purer BeH2 forms from the pyrolysis of di-tert-butylberyllium, Be(C(CH3)3)2 at 210 °C.
A route to highly pure samples involves the reaction of triphenylphosphine, PPh3, with beryllium borohydride, Be(BH4)2:
Be(BH4)2 + 2 PPh3 → BeH2 + 2 Ph3PBH3
Structure
BeH2 is typically formed as an amorphous white solid, but a hexagonal crystalline form with a higher density (~0.78 g cm-3) has been reported, which was produced by heating amorphous BeH2 under pressure with 0.5-2.5% LiH as a catalyst.
In contrast to the flat, hydrogen-bridged, infinite chains previously thought to exist in crystalline BeH2, a more recent study discovered that crystalline BeH2 has a body-centered orthorhombic unit cell containing a network of corner-sharing BeH4 tetrahedra.
According to research, the amorphous form is made up of a network of corner shared tetrahedra.
Chemical properties
- Reaction with water and acids
Beryllium hydride reacts slowly with water but is rapidly hydrolysed by acid such as hydrogen chloride to form beryllium chloride.
BeH2 + 2 H2O → Be(OH)2 + 2 H2
BeH2 + 2 HCl → BeCl2 + 2 H2
- Reaction with Lewis bases
Beryllium hydride reacts with trimethylamine, N(CH3)3 to form a dimeric adduct, with bridging hydrides. However, with dimethylamine, HN(CH3)2 it forms a trimeric beryllium diamide, [Be(N(CH3)2)2]3 and hydrogen. The reaction with lithium hydride, where the hydride ion is the Lewis base, forms sequentially LiBeH3 and Li2BeH4.