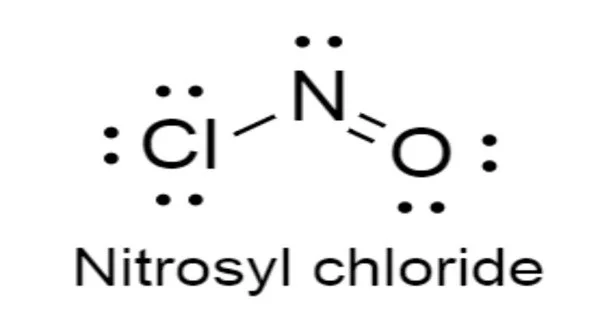
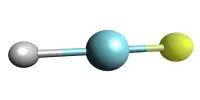
Nitrosyl chloride is a chemical compound having the formula NOCl. At room temperature, it is a yellowish-brown gas with a strong, pungent odor. It is a yellow gas that is usually encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part concentrated nitric acid.
It is a potent electrophile and oxidizing agent. It is also known as Tilden’s reagent after William A. Tilden, who was the first to create it as a pure chemical.
Properties
- Chemical formula: NOCl
- Molar mass: 65.459 g mol−1
- Appearance: yellow gas
- Density: 2.872 mg mL−1
- Melting point: −59.4 °C (−74.9 °F; 213.8 K)
- Boiling point: −5.55 °C (22.01 °F; 267.60 K)
- Solubility in water: Reacts
- Molecular shape: Dihedral, diagonal
Chemical Properties:
Nitrosyl chloride is a source of the nitrosonium cation (NO+), which is often involved in various chemical reactions. It can act as a nitrosating agent, introducing nitroso groups (-NO) into organic compounds.
It is sensitive to moisture and can react with water to produce nitrous acid (HNO2) and hydrochloric acid (HCl):
NOCl + H2O → HNO2 + HCl
Uses
Nitrosyl chloride is largely used as a reagent in chemical synthesis to introduce nitroso groups into organic compounds. It is utilized in the manufacture of some medications and agrochemicals. It is used in the laboratory as a source of nitrosonium ions for nitrosation processes.
Safety
Nitrosyl chloride is a dangerous chemical that must be handled with caution. It is poisonous, and the fumes can irritate the eyes, skin, and respiratory system. When dealing with this combination, proper safety precautions should be used, including the use of suitable protective equipment and working in a well-ventilated location.
Because of the reactivity of nitrosyl chloride and the possibility for harmful gas release, it must be handled with caution and in controlled surroundings, such as a laboratory, by skilled individuals.