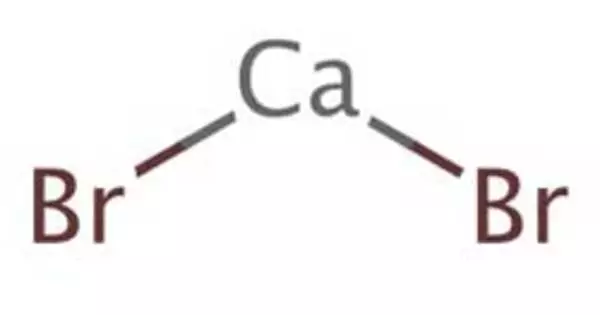
Calcium bromide, also known as calcium dibromide, is a chemical compound that is commonly found in drilling fluids and as a food preservative. Calcium Dibromide is not harmful to any formation. It is both chemically and thermally stable. This compound can be mixed with other solutions containing chlorides and bromides.
Calcium bromide refers to compounds that have the chemical formula CaBr2(H2O)x. Individual compounds include anhydrous (x = 0), hexahydrate (x = 6) and the rare dihydrate (x = 2). It consists of one calcium and two bromine atoms. It is a hygroscopic and colorless crystal with a sharp saline taste in its anhydrous form. All are white powders that dissolve in water and crystallize the hexahydrate from the solutions. The hydrated form is mainly used in some drilling fluids.
Properties
Calcium bromide is a white hygroscopic powder that is available as anhydrous. It has a density of 3.35 grams per liter and a melting point of 730 degrees Celsius. It has a boiling point of 1935 degrees Celsius. Calcium bromide is soluble in water, ethanol, methanol, and acetone, but inorganic solvents are insoluble.
- Chemical formula: CaBr2
- Molar mass: 199.89 g/mol (anhydrous); 235.98 g/mol (dihydrate)
- Appearance: anhydrous is hygroscopic colorless crystals sharp saline taste
- Density: 3.353 g/cm3
- Melting point: 730 °C (1,350 °F; 1,000 K)
- Boiling point: 1,815 °C (3,299 °F; 2,088 K) (anhydrous); 810 °C (dihydrate)
- Solubility in water: 125 g/100 mL (0 °C); 312 g/100 mL (100 °C)
- Solubility in alcohol, acetone: soluble

Synthesis, structure, and reactions
It is made by reacting calcium oxide, calcium carbonate, or calcium metal with hydrobromic acid or elemental bromine. It has a rutile structure with octahedral Ca centers bound to six bromide anions, which also bridge to other Ca centers.
When calcium bromide is strongly heated in air, it reacts with oxygen to form calcium oxide and bromine:
2 CaBr2 + O2 → 2 CaO + 2 Br2
In this reaction the oxygen oxidizes the bromide to bromine.
Calcium bromide can suffer many reactions. It is useful for the chemical industry is the formation of calcium oxide from calcium bromide and oxygen gas. This reaction takes place at high temperatures to produce bromine gas.
Uses
Its primary application is as a dense aqueous solution for drilling fluids. It is also used as a neurosis medication, food preservatives, freezing mixtures, fire retardants, and in photography. It is also used in drilling fluids as dense aqueous solutions. It is also used in the treatment of neurological disorders, freezing mixtures, food preservatives, photography, and fire retardants.
Calcium bromide has been shown to be complex with triphenylphosphine oxide, allowing triphenylphosphine oxide to be removed from reaction mixtures without the use of chromatography.