Caesium fluoride, also known as cesium fluoride, is a hygroscopic white salt and an inorganic compound with the formula CsF. It is an ionic compound that typically appears as a hygroscopic white solid. It is more soluble and dissociates more easily than sodium fluoride or potassium fluoride.
Caesium fluoride can be used as a fluoride anion source in organic synthesis. Caesium also has the highest electropositivity of any non-radioactive element, while fluorine has the highest electronegativity of any element known to science.
Properties
- Chemical formula: CsF
- Molar mass: 151.903 g/mol
- Appearance: white crystalline solid
- Density: 4.64 g/cm3
- Melting point: 703 °C (1,297 °F; 976 K)
- Boiling point: 1,251 °C (2,284 °F; 1,524 K)
- Solubility in water: 573.0 g/100 mL (25 °C)
- Solubility: Insoluble in acetone, diethyl ether, pyridine and ethanol 191 g/100 mL in methanol.
- Crystal structure: cubic, cF8

Synthesis
Caesium fluoride can be made by reacting hydrofluoric acid with caesium hydroxide or caesium carbonate and then removing the water. It can be made by reacting caesium hydroxide (CsOH) with hydrofluoric acid (HF), and the resulting salt can then be purified by recrystallization. The reaction is depicted below:
CsOH + HF → CsF + H2O
Another method for producing caesium fluoride is to react caesium carbonate (Cs2CO3) with hydrofluoric acid, and the resulting salt can then be purified by recrystallization.
Cs2CO3 + 2 HF → 2 CsF + H2O + CO2
In organic solvents, CsF is more soluble than sodium fluoride or potassium fluoride. It is available in anhydrous form, and if water has been absorbed, it is simple to dry in vacuo by heating at 100 °C for two hours. At 825 °C, CsF has a vapor pressure of 1 kilopascal, 10 kPa at 999 °C, and 100 kPa at 1249 °C.
CsF chains as thin as one or two atoms can be grown inside carbon nanotubes.
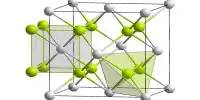
Structure
Caesium fluoride has a halite structure, which means that the Cs+ and F− pack in a cubic closest-packed array as do Na+ and Cl− in sodium chloride.
Applications in organic synthesis
Being highly dissociated, CsF is a more reactive source of fluoride than related salts. CsF is an alternative to tetra-n-butylammonium fluoride (TBAF) and TAS-fluoride (TASF).
- As a base
As with other soluble fluorides, CsF is moderately basic, because HF is a weak acid. The low nucleophilicity of fluoride means it can be a useful base in organic chemistry. CsF gives higher yields in Knoevenagel condensation reactions than KF or NaF.
- Formation of Cs-F bonds
Caesium fluoride is used as a fluoride source in organofluorine chemistry. CsF reacts with hexafluoroacetone in the same way that potassium fluoride does to form a stable perfluoroalkoxide salt. Although potassium fluoride is more commonly used, it will convert electron-deficient aryl chlorides to aryl fluorides (Halex process).
Precautions
CsF is moderately toxic, as are other soluble fluorides. Acid contact should be avoided because it produces highly toxic/corrosive hydrofluoric acid. Caesium ion (Cs+) and caesium chloride are not toxic in general.