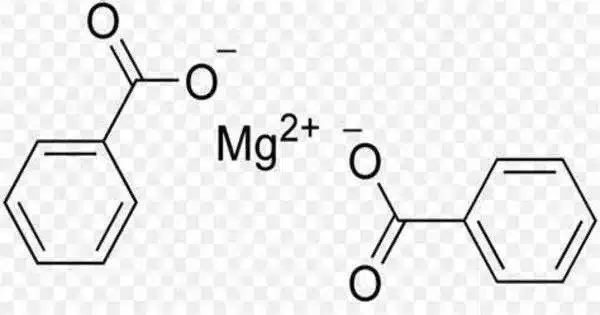
Magnesium benzoate is a chemical compound formed from magnesium and benzoic acid. It was once used to treat gout and arthritis. It is a chemical compound that is formed by the reaction of magnesium hydroxide with benzoic acid. It is used as a food preservative and has the chemical formula Mg(C7H5O2)2.
While magnesium benzoate is an approved food preservative, there are other preservatives available as well, such as sodium benzoate, potassium benzoate, and calcium benzoate. The choice of preservative often depends on factors like the type of food product, pH, and desired shelf life.
Properties
- Appearance: It is typically found in the form of a white crystalline powder.
- Solubility: It is soluble in water and some other polar solvents.
- Chemical Stability: It is chemically stable under normal storage conditions, but it can degrade over time, especially when exposed to high temperatures and humidity.
- Compatibility: It is compatible with a wide range of food products, including beverages, canned goods, salad dressings, and condiments.
Solubility
This compound is soluble in water, which makes it suitable for use in liquid and aqueous food products. It dissolves easily in water, allowing for even distribution throughout the product.
Application
Magnesium benzoate is primarily used as a preservative in various food and beverage products. It helps to extend the shelf life of these products by inhibiting the growth of microorganisms such as bacteria and fungi.
In addition to its preservative properties, magnesium benzoate can also be used as a pH regulator in certain food products. It can help maintain the desired acidity or alkalinity of a product.
Safety
Magnesium benzoate is generally recognized as safe (GRAS) by the U.S. Food and Drug Administration (FDA) when used in accordance with good manufacturing practices. It is considered safe for consumption within specified limits.