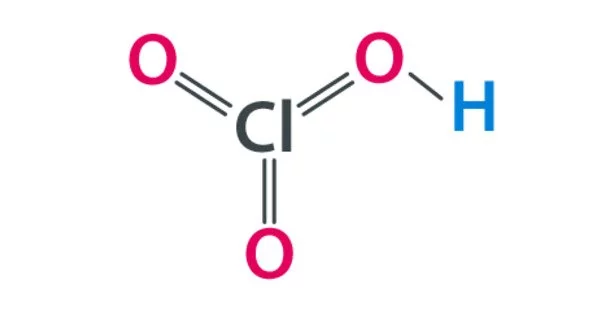
Chloric acid, HClO3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a clear liquid with no color. It is a strong oxidizing agent as well as an acid. It is a strong acid (pKa ≈ −2.7) and an oxidizing agent. When it comes into contact with combustible materials, it increases the likelihood of ignition. It’s a common reagent in chemical analysis and the production of various chemicals.
Chloric acid is a powerful oxidizing agent. It can react with reducing agents, facilitating the transfer of electrons, and promoting oxidation reactions. Its oxidizing properties make it useful in various chemical processes and synthesis reactions.
Properties
Chloric acid is thermodynamically unstable with respect to disproportionation. It is relatively stable under normal conditions. However, it can decompose upon exposure to high temperatures or when in contact with certain substances, such as organic materials or reducing agents. Decomposition of chloric acid can lead to the release of oxygen gas and potentially hazardous situations. It is highly soluble in water, forming a colorless solution. The solubility of chloric acid decreases as the temperature decreases.
- Chemical formula: HClO3
- Molar mass: 4.45914 g mol−1
- Appearance: colourless solution
- Density: 1 g/mL, solution (approximate)
- Solubility in water: >40 g/100 ml (20 °C)
- Acidity (pKa): −2.7
- Conjugate base: Chlorate
- Molecular shape: pyramidal
Chloric acid is stable in cold aqueous solution up to a concentration of approximately 30%, and solution of up to 40% can be prepared by careful evaporation under reduced pressure. Above these concentrations, chloric acid solutions decompose to give a variety of products, for example:
8 HClO3 → 4 HClO4 + 2 H2O + 2 Cl2 + 3 O2
3 HClO3 → HClO4 + H2O + 2 ClO2
Chloric acid is not commonly found in its pure form but can be prepared by the reaction of chlorine dioxide (ClO2) with water. The chemical equation for this reaction is:
2 ClO2 + H2O → 2 HClO3
Production
It can be prepared by the reaction of sulfuric acid with barium chlorate, the insoluble barium sulfate being removed by precipitation:
Ba(ClO3)2 + H2SO4 → 2 HClO3 + BaSO4
Another method is the heating of hypochlorous acid, producing chloric acid and hydrogen chloride:
3 HClO → HClO3 + 2 HCl
Application
Chloric acid is primarily used in the production of other chemicals, such as explosives, dyes, and disinfectants. It can also be used as a laboratory reagent for various chemical reactions. Due to its strong oxidizing properties, chloric acid should be handled with extreme caution, as it can cause severe burns and is toxic when inhaled or ingested.
Hazards
Chloric acid is a powerful oxidizing agent. Most organics and flammables will deflagrate on contact.