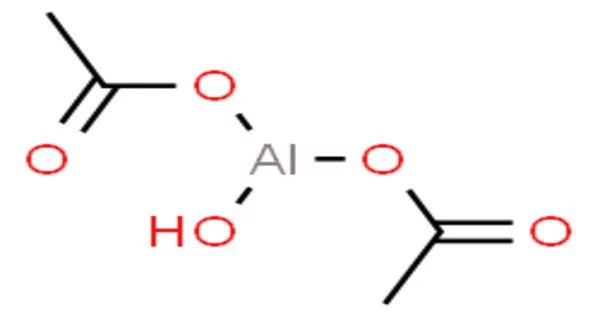
Aluminium diacetate, also known as basic aluminium acetate, is a white powder with the chemical formula C4H7AlO5. It is a salt formed by the reaction of aluminium hydroxide and acetic acid. It is one of several aluminum acetates that can be made by reacting sodium aluminate (NaAlO2) with acetic acid.
Preparations are used topically to treat a variety of skin conditions such as eczema, diaper rash, acne, and so on. It is also used as a mordant in dyeing, a waterproofing agent, and a fireproofing agent. It also serves as a disinfectant in embalming and a dusting powder. It is also used in antiperspirants.
Properties
It is a white, crystalline powder that is soluble in water, forming a neutral solution. It is commonly used as a topical astringent and has antiseptic properties. It is also used in the production of certain medications and as a mordant in dyeing and printing fabrics.
- Chemical formula: C4H7AlO5
- Molar mass: 162.077 g·mol−1
- Appearance: White, opaque crystals
- Molecular weight: 204.114 g/mol
- Complexity: 25.5
- Compound canonicalization: Yes
- Covalent bond: 4
It is a weak acid capable of donating protons to water in order to form hydronium ions. It is generally unreactive with most metals, but it can form metal acetates with certain metals, such as zinc and magnesium. It can form aluminum compounds when it reacts with nonmetals such as chlorine and sulfur. Although it is stable in the presence of most oxidizing agents, it can react with strong oxidizers such as perchloric acid to form aluminum perchlorate. It is stable at room temperature, but at high temperatures it decomposes, releasing water and acetic acid. It is water and glycerol soluble. It dissolves in water at a rate of 4.81 mg/mL.
Applications
Aluminium diacetate is astringent and antiseptic. It is applied topically as a wet dressing, compress, or soak for self-medication to temporarily relieve itching and soothe lesions that are wet or weeping. It soothes skin irritation caused by a variety of factors, including insect bites, athlete’s foot, urushiol-induced contact dermatitis from plants that are toxic to the touch, such as poison ivy, oak, or sumac, and skin irritation caused by sensitivity to soaps, detergents, cosmetics, or jewelry. It is also used to treat bruise swelling.
In the dyeing industry, basic aluminium diacetate is used as a mordant for fibers such as cotton in conjunction with aluminum triacetate.