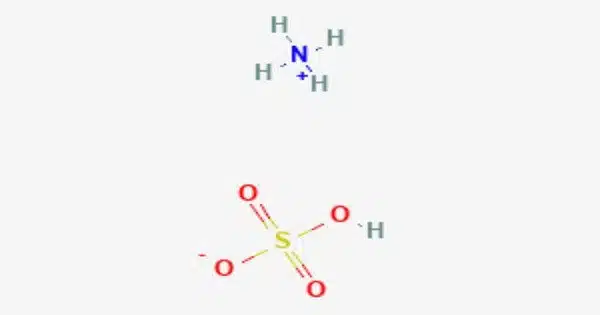
Ammonium bisulfate, also known as ammonium hydrogen sulfate, is a white, crystalline solid with the formula (NH4)HSO4. It is typically found as a white crystalline solid or in granular form. This salt is the product of the half-neutralization of sulfuric acid by ammonia. It is highly soluble in water.
The solubility increases with temperature. It is acidic in nature. It can release hydrogen ions (H+) in water, leading to an acidic solution. It may have a slight ammonia odor due to the presence of ammonium ions.
Properties
- Chemical formula: (NH4)HSO4
- Molar mass: 115.11 g/mol
- Appearance: White solid
- Density: 1.78 g/cm3
- Melting point: 147 °C (297 °F; 420 K)
- Solubility in water: Very soluble
- Solubility in other solvents: Soluble in methanol; insoluble in acetone
Production
It is commonly collected as a byproduct of the “acetone cyanohydrin route” to the commodity chemical methyl methacrylate.
It can also be obtained by hydrolysis of sulfamic acid in aqueous solution, which produces the salt in high purity:
H3NSO3 + H2O → [NH4]+[HSO4]−
It also arises by the thermal decomposition of ammonium sulfate:
(NH4)2SO4 → (NH4)HSO4 + NH3
Applications
Ammonium bisulfate is used to adjust the pH of solutions in various industries, such as water treatment and textile processing. It is employed as a cleaning agent for metal surfaces. It can be used as a fertilizer additive to provide a source of ammonium nitrogen and sulfur to plants. It may be used as a laboratory reagent in certain chemical experiments and analyses.
It can be further neutralized with ammonia to form ammonium sulfate, a valuable fertilizer. It can be used as a weaker alternative to sulfuric acid, although sodium bisulfate is much more common.
Natural occurrence
A related compound of the (NH4)3H(SO4)2 formula, occurs as the rare mineral letovicite, known from coal fire environments.
Safety
As with any chemical, proper safety precautions should be taken when handling ammonium bisulfate. This includes using appropriate personal protective equipment (PPE) and following established safety protocols. It should be stored in a cool, dry place away from incompatible materials. Containers should be tightly sealed to prevent moisture absorption. It may release irritating or toxic fumes when heated, so proper ventilation is essential.