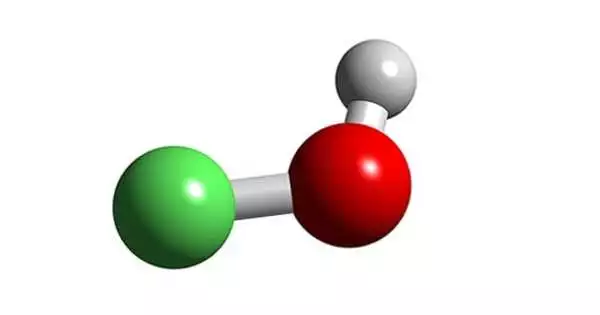
Hypofluorous acid, chemical formula HOF, is the only known oxyacid of fluorine and the only known oxoacid in which the main atom gains electrons from oxygen to create a negative oxidation state. It’s a chemical compound with the formula HOF. In hypofluorites, the oxygen oxidation state is 0. It is an unstable and highly reactive compound made up of hydrogen, oxygen, and fluorine atoms. It’s also the only hypohalous acid that can be isolated as a solid.
Hypofluorous acid is part of the group of oxyacids, which are acids that contain oxygen. Due to its high reactivity and instability, hypofluorous acid is not commonly encountered in pure form and is primarily studied in specialized laboratories. It can decompose or react violently under various conditions, making it challenging to handle safely.
Properties
- Chemical formula: HOF
- Molar mass: 36.0057 g mol−1
- Appearance: pale yellow liquid above −117 °C
- White solid below −117 °C
- Melting point: −117 °C (−179 °F; 156 K)
- Boiling point: decomposes at 0 °C
Reaction
The main application of hypofluorous acid is in fluorination reactions, where it can introduce fluorine atoms into organic and inorganic molecules. These reactions are used in the synthesis of various fluorine-containing compounds. The reactivity of hypofluorous acid stems from the weak hydrogen-oxygen bond, which is easily broken to release reactive oxygen and fluorine species.
HOF is an intermediate in the oxidation of water by fluorine, which produces hydrogen fluoride, oxygen difluoride, hydrogen peroxide, ozone and oxygen. HOF is explosive at room temperature, forming HF and O2:
2 HOF → 2 HF + O2
It was isolated in the pure form by passing F2 gas over ice at −40 °C, collecting the HOF gas, and condensing it:
F2 + H2O → HOF + HF
The compound has been characterized in the solid phase by X-ray crystallography as a bent molecule with an angle of 101°. The O–F and O–H bond lengths are 144.2 and 96.4 picometres, respectively. The solid framework consists of chains with O–H···O linkages. The structure has also been analyzed in the gas phase, a state in which the H–O–F bond angle is slightly narrower (97.2°).
Rozen’s reagent is a solution of hypofluorous acid in acetonitrile that is generated in situ by passing gaseous fluorine through acetonitrile that contains water. Because of its reactivity, handling hypofluorous acid necessitates specialized equipment and precautions to ensure safety. Due to its instability, it is frequently generated in situ (on-site) and used immediately in reactions. Researchers and chemists who work with hypofluorous acid must be well-trained in the handling of hazardous chemicals and must adhere to strict safety protocols.
Overall, hypofluorous acid is an intriguing compound in the realms of chemical synthesis and fluorination chemistry, but its high reactivity and instability make it a difficult substance to work with.