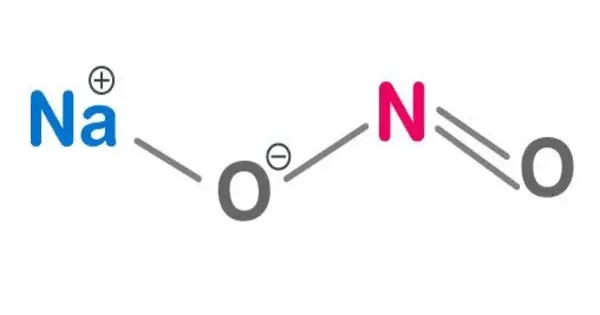
Sodium nitrite has the chemical formula NaNO2 and is an inorganic compound. It has the appearance of a yellowish white crystalline solid. It is a hygroscopic white to slightly yellowish crystalline powder that is very soluble in water. It is the most important nitrite salt from an industrial standpoint. It is a precursor to a wide range of organic compounds, including pharmaceuticals, dyes, and pesticides, but it is best known as a food additive found in processed meats and (in some countries) fish products.
Properties
An inorganic salt is sodium nitrate. It is a solid white salt that is a strong oxidizing agent and is used as a preservative due to its ability to keep bacteria from colonizing food. Let us learn more about the sodium nitrite formula, as well as its chemical structure, properties, and applications, in this brief article.
- Appearance: White crystalline solid
- Chemical Formula: NaNO2
- Melting Point: 271 °C
- Boiling Point: Decomposes above 320 °C
- Density: 2.17 g/cm³
- Molar Mass: 68.9953 g/mol
- Solubility in Water: Soluble

Production
The industrial production of sodium nitrite involves one of two processes: the reduction of nitrate salts or the oxidation of lower nitrogen oxides. One method uses molten sodium nitrate as the salt and oxidized lead, whereas a more modern method uses scrap iron filings to reduce the nitrate.
A more commonly used method involves the general reaction of nitrogen oxides in alkaline aqueous solution with the addition of a catalyst. The exact conditions depend on which nitrogen oxides are used and what the oxidant is, as the conditions must be carefully controlled to avoid over-oxidation of the nitrogen atom. Sodium nitrite has also been produced by reducing nitrate salts with heat, light, ionizing radiation, metals, hydrogen, and electrolytic reduction.
Preparation
There are many ways to produce Sodium Nitrite.
Sodium Nitrite can be obtained by reacting nitrous acid with sodium hydroxide.
2 NaOH + N2O3 ⇢ 2 NaNO2 + H2O
The thermal reduction of Sodium Nitrate with calcium sulfite will also yield sodium nitrite.
NaNO3 + CaSO3 ⇢ NaNO2 + CaSO4
Uses
Industrial chemistry
Sodium nitrite is primarily used in the industrial production of organonitrogen compounds. It is a reagent used to convert amines into diazo compounds, which are important precursors to many dyes, including diazo dyes. Nitrites are used to make nitroso compounds. These are commonly found in the rubber industry.
It is used for phosphatizing and detinning in a variety of metallurgical applications. Sodium nitrite is a corrosion inhibitor that is used as an additive in industrial greases, as an aqueous solution in closed loop cooling systems, and as a heat transfer medium in a molten state.
Food additive and preservative
Sodium nitrite is used to accelerate the curing of meat while also imparting an appealing pink color. Nitrite reacts with meat myoglobin to cause color changes, first converting to nitrosomyoglobin (bright red), then to nitrosohemochrome upon heating (a pink pigment).
Historically, salt has been used to preserve meat. The color of the salt-preserved meatproduct was usually brownish-gray. When sodium nitrite is mixed with salt, the meat turns red, then pink, resembling cured meats such as ham, bacon, hot dogs, and bologna.
Medication
To treat cyanide poisoning, sodium nitrite is combined with sodium thiosulfate as a medication. It is only advised in severe cases of cyanide poisoning. Sodium thiosulfate alone is usually recommended for those who have both cyanide and carbon monoxide poisoning. It is administered through a slow injection into a vein.
Toxicity
Sodium nitrite is poisonous. The LD50 in rats is 180 mg/kg, and the LD50 in humans is 71 mg/kg. However, death from sodium nitrite ingestion can occur at lower doses. Sodium nitrite is occasionally used in homicide. Since 2019, the global online marketplace eBay has prohibited the sale of sodium nitrite. To avoid accidental intoxication, sodium nitrite (blended with salt) sold as a food additive in the United States is bright pink to avoid being misidentified as plain salt or sugar. Nitrited curing salt is not dyed in other countries, but it is strictly regulated.