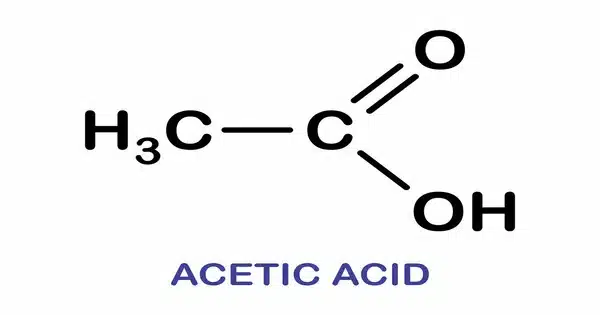
Acetic acid is a colorless, acidic liquid with the chemical formula CH3COOH (also known as CH3CO2H, C2H4O2, or HC2H3O2). The liquid is clear and colorless, with a strong, unpleasant odor. It is a weak acid that is among the simplest carboxylic acids. Vinegar contains at least 4% acetic acid by volume, making it the primary component of vinegar other than water. It has been used in vinegar since at least the third century BC.
Acetic acid is the second-simplest carboxylic acid (after formic acid). It is an essential chemical reagent and industrial chemical in a variety of applications, primarily in the manufacturing of cellulose acetate for photographic film, polyvinyl acetate for wood glue, and synthetic fibers and fabrics. Diluted acetic acid is a common descaling agent in residences. Acetic acid is regulated in the food industry under the food additive code E260 as an acidity regulator and condiment. In biochemistry, the acetyl group, produced from acetic acid, is essential to all forms of life. When linked to coenzyme A, it plays an important role in the metabolism of carbohydrates and lipids.
Acetic acid, which is generated from methanol, has a global demand of around 6.5 million metric tons per year (t/a). Its manufacture and subsequent industrial use endangers workers’ health, including incidental skin damage and chronic respiratory problems caused by inhalation.
Properties
- Chemical formula: CH3COOH
- Molar mass: 60.052 g·mol−1
- Appearance: Colourless liquid
- Odor: Heavily vinegar-like
- Density: 1.049 g/cm3 (liquid); 1.27 g/cm3 (solid)
- Melting point: 16 to 17 °C; 61 to 62 °F; 289 to 290 K
- Boiling point: 118 to 119 °C; 244 to 246 °F; 391 to 392 K
- Solubility in water: Miscible
- Vapor pressure: 1.54653947 kPa (20 °C); 11.6 mmHg (20 °C)
Production
Acetic acid is manufactured industrially through both synthetic and bacterial fermentation. The carbonylation of methanol produces approximately 75% of the acetic acid used in the chemical industry. The biological method accounts for only about 10% of global production, but it is nevertheless crucial for vinegar manufacturing because many food purity rules require vinegar used in foods to be biologically produced. Other processes include isomerizing methyl formate, converting syngas to acetic acid, and oxidizing ethylene and ethanol in the gas phase.
Uses
- Food Industry: Acetic acid is widely used in the food industry as a flavoring agent, preservative, and pH regulator. It is a key component of vinegar.
- Chemical Industry: It serves as a precursor to various chemicals and plastics. It’s used in the production of vinyl acetate monomer (VAM), which is a key ingredient in the production of polyvinyl acetate (PVA) and other polymers.
- Pharmaceuticals: Acetic acid is used in the production of various pharmaceuticals and drugs.
- Cleaning: Due to its acidic properties, acetic acid is used in household cleaning products, such as glass cleaners and disinfectants.
Health Considerations
- Ingestion of concentrated acetic acid can cause irritation and damage to the gastrointestinal tract.
- Inhalation of acetic acid vapors can irritate the respiratory tract.
- Exposure to concentrated acetic acid can cause severe burns to the skin and eyes.
However, acetic acid is generally recognized as safe (GRAS) by regulatory agencies when used in food products in appropriate concentrations. Overall, It plays a significant role in various industries and everyday applications due to its unique properties and versatile nature.