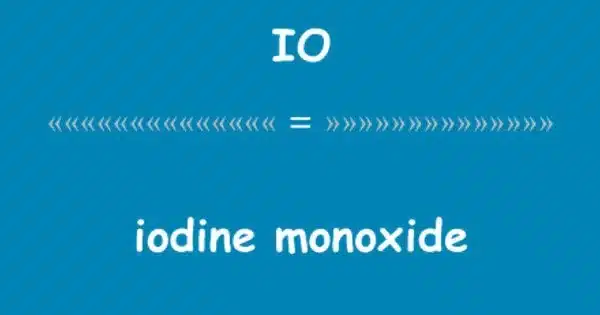
Iodine monoxide has the chemical formula IO• and is a binary inorganic compound of iodine and oxygen. It is a chemical compound made up of iodine (I) and oxygen (O). It is a diatomic molecule, which means it is made up of two atoms. This compound, a free radical, is the most basic of many iodine oxides. It belongs to a class of compounds formed by the reaction of halogens (such as iodine) with oxygen. It is related to the radicals oxygen monofluoride, chlorine monoxide, and bromine monoxide.
In the laboratory, iodine monoxide can be produced using a variety of methods, including the reaction of iodine with oxygen gas. At room temperature, it is typically a dark brown solid with a distinct odor. This compound is not as well-known as some other halogen oxides, but it has been studied for its reactivity and properties.
Properties
- Physical State: It is typically found as a dark brown to black solid or a dark red-brown gas, depending on its form and conditions.
- Stability: IO is not very stable and tends to decompose, especially at higher temperatures. It is sensitive to light and can decompose when exposed to ultraviolet radiation.
- Chemical Reactivity: IO is a reactive compound and can participate in various chemical reactions. It can react with other compounds containing oxygen or hydrogen, forming different iodine and oxygen-containing species.
Preparation
It can be prepared by the reaction of iodine with oxygen. The reaction is often carried out in the presence of a catalyst or under specific conditions to favor IO formation.
Synthesis
Iodine monoxide can be obtained by the reaction between iodine and oxygen:
I2 + O2 → 2 IO
Chemical properties
Iodine monoxide decomposes to its prime elements:
2 IO → I2 + O2
Iodine monoxide reacts with nitric oxide:
2 IO + 2 NO → I2 + 2 NO2
Atmosphere
Atmospheric iodine atoms (e.g. from iodomethane) can react with ozone to produce the iodine monoxide radical:
I2 + 2 O3 → 2 IO + 2 O2
This process can contribute to ozone depletion. IO is of interest in atmospheric chemistry, particularly in the study of iodine compounds in the atmosphere. It is involved in the cycling of iodine in the Earth’s atmosphere and plays a role in the formation of aerosols.
Uses
Iodine monoxide is not commonly used for specific practical applications. It is more often studied for its reactivity and its role in atmospheric chemistry.