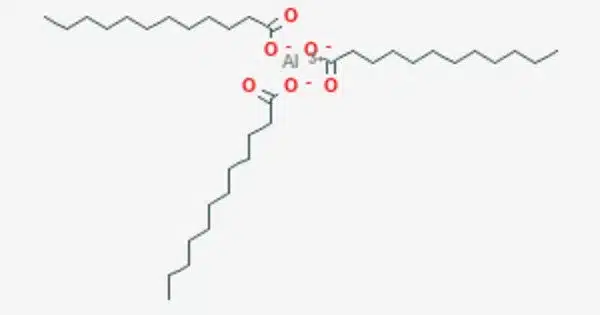
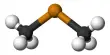
Aluminium laurate is a metal-organic compound with the chemical formula C36H69AlO6. It is a chemical compound that is a salt of lauric acid and aluminum. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid (lauric acid). Lauric acid is a saturated fatty acid commonly found in coconut oil and palm kernel oil. When lauric acid reacts with aluminum, it forms a compound known as an aluminum laurate.
Aluminum laurate may have various applications, but it is not as widely recognized or studied as some other aluminum compounds. Aluminum compounds are often used in various industries, including pharmaceuticals, cosmetics, and as catalysts in organic synthesis.
Properties
Aluminum laurate is likely to be a salt or complex formed by the reaction of aluminum with lauric acid, which is a fatty acid commonly found in coconut oil and palm kernel oil. Laurates, in general, are esters or salts of lauric acid.
- Molar mass: 624.9
- Appearance: White powder
- Boiling point: 296 °C (565 °F; 569 K)
- Solubility in water: Soluble
- State: It can exist in various states, including solid, liquid, or powder form, depending on the specific compound.
- Reactivity: It can be reactive, particularly with acids. They may undergo reactions such as hydrolysis in water.
- Acid-Base Properties: Depending on the conditions, it may exhibit amphoteric behavior, meaning they can act as both acids and bases.
Use
Aluminium laurate is used as an anticaking agent, free-flow agent, or emulsifier. It finds applications in various industries, including pharmaceuticals, cosmetics, and as additives in some industrial processes.
Toxicity and Safety
Some aluminum compounds may pose health concerns, especially if exposure is excessive. It’s important to consider safety guidelines and regulations when handling these compounds. It may have environmental implications, and their disposal and use should be in accordance with environmental regulations.