Extraction of Copper
Copper pyrites is the main ore. The method used to extract copper from its ores depends on the nature of the ore. Sulphide ores such as chalcopyrite are converted to copper by a different method from silicate, carbonate or sulphate ores. The extraction process involves the following steps:
(i) Condensation of ore: Only 2≈3% Copper (Cu) is present in copper pyrites (CuFeS2). After concentrating it by oil-froth floatation process, the amount of Cu will be 20≈30%. copper pyrites) and similar sulphide ores are the commonest ores of copper. The ores typically contain low percentages of copper and have to be concentrated by, for example, froth flotation before refining.
(ii) Roasting: The final mixture obtained is known as Calcine which contains 40% Cu.
(iii) Smelting: The product is called Copper Matt which contains 50% Cu. Most of the sulphur in the chalcopyrite turns into sulphur dioxide gas. This is used to make sulphuric acid via the Contact Process.
(iv) Bessemarization: The final product obtained by self-midation of Cu is known as Blister Copper that contains 97% Cu.
2Cu2S + 3O2 → 2Cu2O (partly) + 2SO2
2Cu2O + Cu2S → 5 Cu + SO2 [Self-Midation reaction]
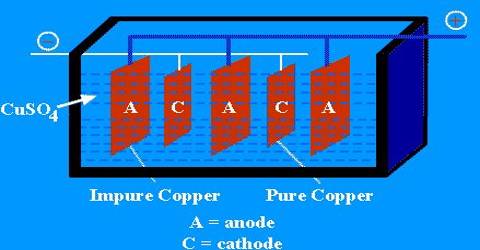
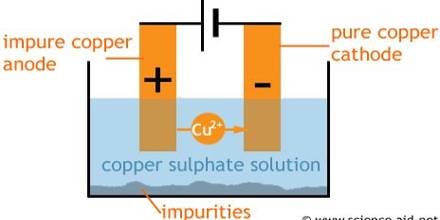
The end product of this is called blister copper – a porous brittle form of copper, about 98 – 99.5% pure. Purification of blister Copper by Electrolysis in CuSO4 solution. The obtained Cu is 99.99% pure.