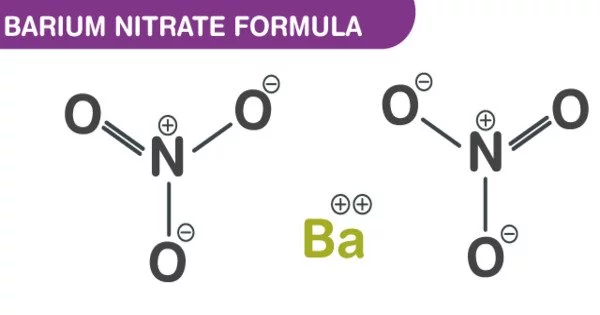
The inorganic compound barium nitrate has the chemical formula Ba(NO3)2. It has the appearance of a white crystalline solid. It is colorless, toxic, and water-soluble, like most barium salts. It has a green flame and is an oxidizer; it is commonly used in pyrotechnics.
Noncombustible, but hastens the combustion of combustible materials. An explosion may occur if large quantities of combustible material are involved in the fire or the combustible material is finely divided. It may explode if exposed to heat or fire for an extended period of time. Nitrogen oxides that are toxic when burned.
Properties
It has the appearance of a crystalline solid white. It is a noncombustible compound that improves the combustion of combustible elements. It has a green flame and is an excellent oxidizing agent. It’s commonly used in pyrotechnics.
- Chemical formula: Ba(NO3)2
- Molar mass: 261.337 g/mol
- Appearance: white, lustrous crystals
- Odor: odorless
- Density: 3.24 g/cm3
- Melting point: 592 °C (1,098 °F; 865 K) (decomposes)
- Solubility in water: 4.95 g/100 mL (0 °C); 34.4 g/100 mL (100 °C)
- Solubility: slightly soluble in acetone, and ethanol
- Crystal structure: cubic

Production of Barium nitrate
Barium dinitrate is produced by two processes that start with the source component for barium viz carbonate.
Method 1:
- Dissolve barium carbonate (BaCO3) in nitric acid (HNO3)
- Allow the precipitation of iron impurities
- Separate the impurities by filtration
- Evaporation
- Crystallization.
Method 2:
It is obtained by combining barium sulfide (BaS) with nitric acid (HNO3). At higher temperatures, barium nitrate (Ba(NO3)2) decomposes to barium oxide (BaO):
2Ba(NO3)2 → 2BaO + 4NO2 + O2
Manufacture, occurrence, and reactions
Barium nitrate is produced through two processes that begin with the main source of barium, carbonate. The first step is to dissolve barium carbonate in nitric acid, allowing any iron impurities to precipitate before filtering, evaporateing, and crystallizing. The second method involves combining barium sulfide and nitric acid.
It occurs naturally as the very rare mineral nitrobarite.
At elevated temperatures, barium nitrate decomposes to barium oxide:
2Ba(NO3)2 → 2BaO + 4NO2 + O2
Applications
- Barium nitrate is used in the production of BaO-containing materials.
- It is used in the production of barium oxide containing materials.
- Used in green signal lights.
- Used in making ceramic glazes.
- Used as a rodenticide.
- Used in detonators.
- Used in primers and tracer bullets.
- Used in the making of paints.
- Used as an oxidizing agent.
- Used in making explosives.
Safety
Barium nitrate, like all soluble barium compounds, is toxic when consumed or inhaled. As first aid for barium poisoning, sulfate salt solutions such as Epsom salts or sodium sulfate may be administered because they precipitate the barium as the insoluble (and non-toxic) barium sulfate. Inhalation can also irritate the respiratory tract. While contact with the skin or eyes is less dangerous than ingestion or inhalation, it can still cause irritation, itching, redness, and pain.
Occupational exposure limits have been set at 0.5 mg/m3 over an eight-hour time-weighted average by the Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health.