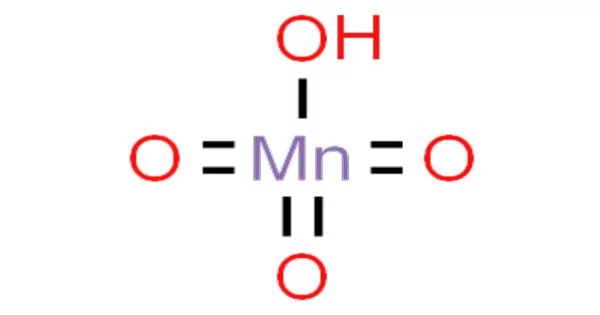
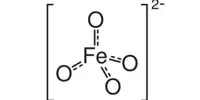
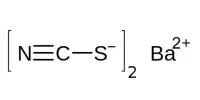Permanganic acid is an inorganic chemical having the formula HMnO4. It is a hypothetical acid generated from permanganate, an inorganic anion. This potent oxoacid has been isolated as its dihydrate. In actuality, it is exceedingly unstable and has never been isolated or observed in its pure form. It is highly unstable and decomposes quickly, especially in the presence of organic matter or reducing chemicals.
It is the conjugate acid of permanganate salts. It has received very few publications, and both its characterization and applications are very limited. It is an example of a “noble” or “metastable” acid, which means it cannot survive under normal conditions and rapidly decomposes into other molecules.
Properties
- Chemical formula: HMnO4
- Molar mass: 119.94 g mol−1
- Appearance: Violet
- Acidity (pKa): about -4.6 to -2.3
- Conjugate base: Permanganate
Preparation and structure
Permanganic acid is typically produced by reacting dilute sulfuric acid with a solution of barium permanganate, with the insoluble barium sulfate byproduct filtered out:
Ba(MnO4)2 + H2SO4 → 2 HMnO4 + BaSO4↓
The sulfuric acid employed must be dilute; reactions of permanganates with strong sulfuric acid produce manganese heptoxide as anhydride.
Permanganic acid has also been synthesized by reacting hydrofluorosilicic acid with potassium permanganate, electrolysis, and hydrolysis of manganese heptoxide, however, the last method frequently ends in explosions.
Crystalline permanganic acid has been prepared at low temperatures as the dihydrate, HMnO4·2H2O.
Although its structure has not been verified spectroscopically or crystallographically, HMnO4 is assumed to be adopt a tetrahedral structure akin to that of perchloric acid.
Reactions
HMnO4 is deprotonated as a strong acid to create the brightly purple permanganates. Potassium permanganate, KMnO4, is a common, versatile, and potent oxidizing agent.
Permanganic acid solutions are unstable and eventually decompose into manganese dioxide, oxygen, and water, with manganese dioxide initially created stimulating further decomposition. Heat, light, and acids all hasten decomposition. Concentrated solutions degrade faster than dilute solutions.
Application
Although permanganic acid is not directly employed due to its instability, permanganates such as potassium permanganate (KMnO4) are widely used in a variety of applications such as water treatment, chemistry, and medicine. They are employed in redox titrations, disinfectants, and oxidizers.
Potassium permanganate (KMnO4) is the most common and stable form of permanganate, and it is frequently utilized as an oxidizing agent in a variety of chemical reactions. Permanganate ions (MnO4-) are strong oxidizing agents that play an important role in many redox processes in chemistry.