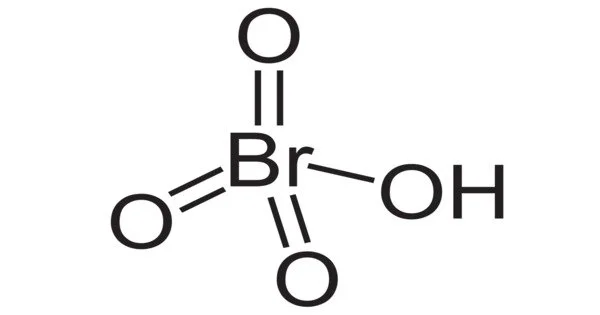
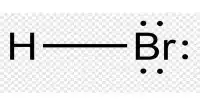Perbromic acid is an inorganic compound with the formula HBrO4. It is an oxoacid of bromine. It is a bromine oxoacid. It is a perbromate conjugate acid. It is unstable and cannot be created by removing chlorine from perchloric acid, as periodic acid is; it can only be created by protonating the perbromate ion. Bromic acid is an oxoacid of bromine. It is a bromate conjugate acid. The color of the solution changes to yellow during its decomposition to bromine at room temperature. It is a strong oxidizing agent.
Perbromic acid is a strong acid that oxidizes rapidly. It is the least stable halogen(VII) oxoacid. It quickly decomposes to bromic acid and oxygen when exposed to air. It reacts with bases to form perbromate salts.
Properties
The molecular weight of the compound is 144.908 g/mol. Because of their instability, their melting and boiling points were calculated using computational methods, yielding values of 204.77° C and 512.23° C, respectively. Its solubility in water is also calculated to be in the order of 1 x 10 6 Mg per liter at 25°C. Perbromic acid is a strong acid that contains one proton for every heptavalent bromine atom.
- Chemical formula: BrHO4
- Molar mass: 144.908
- Melting point: decomposes before melting, unstable as solid
- Conjugate base: Perbromate
Compound
The compound is a bromine oxacid acid with an oxidation state of 7+. As the perbromic acid is prepared, it cannot be formed by the displacement of chlorine from the perchloric acid; it can only be done by protonation of the perbromat ion.
Application
Perbromic acid is primarily used in the laboratory as a reducing agent. Despite its high potential REDOX (+1.76 volts), dilute solutions of perbromic acid are slow oxidizing agents. However, it is a better oxidant than perchloric acid. Bromide and iodide ions can be slowly oxidized by perbromic acid. In the presence of nitric acid, solutions of 12 molar concentration can rapidly oxidize the chloride ion and explode. Concentration solutions 3 molar perbric acid can easily oxidize stainless steel.
Reactivity and hazards
Perbromic acid is an unstable compound however it has strong acid properties when it is isolated. Extremely dangerous in case of skin contact (corrosive and irritant), eye contact (irritant), and in case of ingestion. Also very dangerous in case of inhalation.
Severe overexposure may result in lung damage, asphyxia, loss of consciousness or death. Prolonged exposure may cause skin burns and ulcerations. Overexposure by inhalation may cause respiratory irritation.
Redness, watering, and itching are symptoms of eye inflammation. Itching, peeling, redness, and, on rare occasions, blistering are symptoms of skin inflammation. The substance is extremely harmful to the kidneys, lungs, and mucous membranes. Repeated or prolonged exposure to the substance may result in organ damage.
If they come into contact with your eyes, check to see if they are wearing contact lenses and remove them right away. For at least 15 minutes, flush the eyes with tap water while keeping the eyelids open. You can use cold water. No eye ointment should be used.