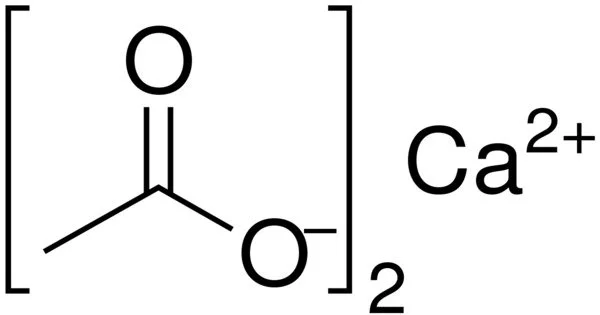
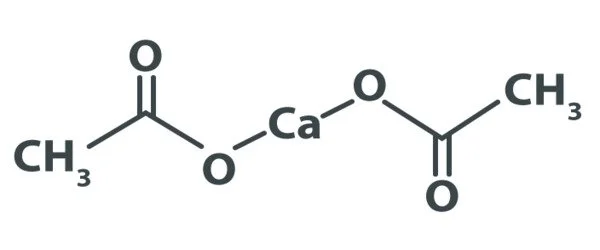
A calcium salt of acetic acid, calcium acetate is a chemical compound. It is an acetic acid calcium salt. Ca(C2H3O2)2 is the formula for it. Calcium acetate is the common name, while calcium ethanoate is the scientific name. Acetate of lime is an older name. Because the anhydrous form is very hygroscopic, the monohydrate (Ca(CH3COO)2•H2O) is the most common.
Calcium is a mineral that is required for a variety of cellular functions such as nerve impulse transmission, muscle contraction, cardiac function, bone formation, and capillary and cell membrane permeability. Because of its phosphate-binding properties, calcium acetate is given orally to prevent or treat calcium deficiency as well as hyperphosphatemia.
Properties
It is a colorless and white crystalline solid having a slight odor of acetic acid. It can be dissolved in water and alcohol and but it is insoluble in benzene and acetone. In its anhydrous form, it is strongly hygroscopic and therefore occurs as monohydrate in its common form.
- Chemical formula: C4H6CaO4
- Molar mass: 158.166 g·mol−1
- Appearance: White solid, hygroscopic
- Odor: slight acetic acid odor
- Density: 1.509 g/cm3
- Melting point: 160 °C (320 °F; 433 K) decomposition to CaCO3 + acetone
- Solubility in water: 37.4 g/100 mL (0 °C); 29.7 g/100 mL (100 °C)
- Solubility: slightly soluble in methanol, and hydrazine; insoluble in acetone, ethanol, and benzene
Production
Calcium acetate can be prepared by soaking calcium carbonate (found in eggshells, or in common carbonate rocks such as limestone or marble) or hydrated lime in vinegar:
CaCO3(s) + 2CH3COOH(aq) → Ca(CH3COO)2(aq) + H2O(l) + CO2(g)
Ca(OH)2(s) + 2CH3COOH(aq) → Ca(CH3COO)(aq) + 2H2O(l)
Since both reagents would have been available pre-historically, the chemical would have been observable as crystals then.
Uses
Calcium acetate is a calcium supplement used to control the level of phosphate in the blood for patients on dialysis due to severe kidney disease. It is used to control phosphate levels to keep them from getting too high in people with kidney failure who are on dialysis.
Phosphate levels in the blood may rise (called hyperphosphatemia) in kidney disease, causing bone problems. Phosphate in the diet is bound by calcium acetate, lowering blood phosphate levels. Calcium acetate is a food additive that is used as a stabilizer, buffer, and sequestrant, primarily in candy products under the code E263.
Traditionally, tofu is made by coagulating soy milk with calcium sulfate. Calcium acetate has been discovered to be a better alternative because it is soluble and therefore requires less skill and a smaller amount. Prior to the development of the cumene process, calcium acetate was a common starting material for the synthesis of acetone due to its low cost:
Ca(CH3COO)2 → CaCO3(s) + (CH3)2CO
- It is used as a food additive.
- Used as a corrosion inhibitor.
- Used is the manufacturing of lubricants.
- Used in bakery goods as an anti-mold agent.
- Used as an esterification catalyst.
- Used in the making of agricultural products.
Occurrence in nature
Pure calcium acetate is a mineral that has yet to be discovered. Calclacite — calcium acetate chloride pentahydrate — is listed as a known mineral, but its origins are likely anthropogenic (man-made, rather than naturally occurring), and it may be debunked soon.