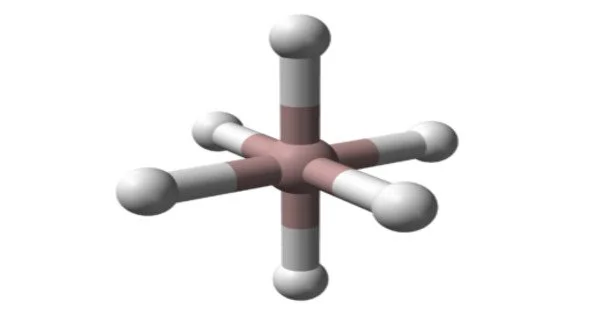
Aluminum hydride appears as a colorless to white solid. It is a white, pyrophoric powder that is highly reactive with water and air. It is a covalent compound, and it is classified as a group 13 hydride.
Aluminium hydride (also known as alane and alumane) is an inorganic compound with the formula AlH3. Alane and its derivatives are common reducing (hydride addition) reagents in organic synthesis that are used in solution at both the laboratory and industrial scales. In solution, typically in etherial solvents such as tetrahydrofuran or diethyl ether, aluminum hydride forms complexes with Lewis bases and reacts selectively with specific organic functional groups (e.g., with carboxylic acids and esters over organic halides and nitro groups), and although it is not a preferred reagent, it can react with carbon-carbon multiple bonds (i.e., through hydroalumination).
Properties
- Highly Reactive: Alane is a highly reactive compound and can react violently with water, acids, or oxidizing agents. It must be handled with extreme care and under an inert atmosphere.
- Strong Reducing Agent: Alane is a strong reducing agent and can reduce many metal ions to their respective metals. This property has made it useful in several industrial applications, such as reducing metal oxides to pure metals.
- Solid at Room Temperature: Alane is a white, crystalline solid at room temperature, with a melting point of 150°C and a boiling point of 129°C. It is insoluble in water but soluble in many organic solvents.
- High Energy Density: Alane has a very high energy density, making it a potential candidate for hydrogen storage in fuel cells. However, its high reactivity and instability make it challenging to store and handle.
- Strong Lewis Acid: Alane is a strong Lewis acid, meaning it can accept a pair of electrons from a Lewis base. This property makes it useful as a catalyst in several organic reactions.
- Air Sensitive: Alane is air-sensitive and reacts with oxygen and moisture in the air, forming aluminum oxide and hydrogen gas. Therefore, it must be stored and handled under an inert atmosphere.

Preparation
Aluminium hydride can be prepared by reacting aluminum powder with hydrogen gas at high temperatures and pressures. It is also formed as a byproduct of the reaction between aluminum and lithium hydride.
Aluminium hydride is a powerful reducing agent and can react violently with water, releasing hydrogen gas. It is used in organic synthesis as a reducing agent and is also a component in rocket propellants. However, its high reactivity and sensitivity to air and moisture make it challenging to handle and store safely. In addition to its reactivity, another limitation of aluminum hydride is its cost. It is relatively expensive to produce and is not widely used due to the availability of less expensive reducing agents.
Applications
Aluminum hydride is commonly used as a reducing agent in organic chemistry and can reduce a wide range of functional groups, including ketones, aldehydes, esters, and carboxylic acids. It can also be used in hydrogen storage systems, as it has a high hydrogen content and can release hydrogen upon heating.
However, aluminum hydride is also highly reactive and can be dangerous to handle. It is sensitive to air and moisture and can spontaneously ignite in the presence of water or air. Therefore, it is typically handled under inert conditions and with appropriate safety precautions.