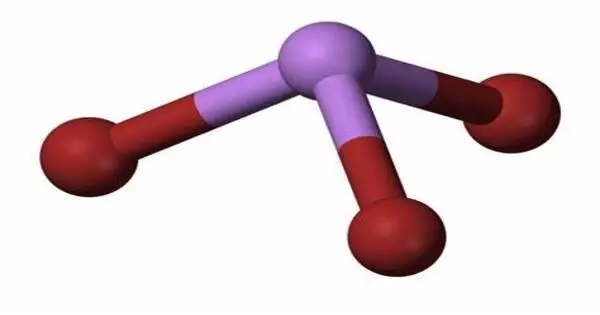
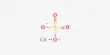
Arsenic tribromide (AsBr3) is an inorganic chemical that is a bromide of arsenic. It is a yellow, oily liquid that is extremely poisonous and strongly reacts to water. Arsenic is a chemical element with the symbol As and atomic number 33. This pyramidal molecule is the sole known binary arsenic bromide. AsBr3 is notable for its extremely high refractive index of around 2.3. It also has an extremely high diamagnetic susceptibility. It is largely utilized as a reagent in organic synthesis, particularly for the production of organoarsenic compounds.
It is a toxic metalloid that appears in a variety of allotropic forms, including yellow (molecular non-metallic) and numerous black and gray forms (metalloids), orthorhombic prisms, and colorless rhombic crystals. Bromine is a halogen element with the symbol Br and the atomic number 35. Diatomic bromine does not exist naturally, however bromine salts can be found in crustal rock.
Properties
- Physical State: It is a solid at room temperature. It typically appears as a yellowish-brown crystalline solid.
- Odor: It has a pungent odor, reminiscent of hydrogen bromide.
- Solubility: It is soluble in non-polar solvents such as carbon tetrachloride and chloroform, but insoluble in water.
- Melting and Boiling Point: It has a melting point of around 31.5°C and a boiling point of approximately 220°C.
Preparation
Arsenic tribromide can be prepared by the direct bromination of arsenic powder. Alternatively, arsenic(III) oxide can be used as the precursor in the presence of elemental sulfur:
2 As2O3 + 3 S + 6 Br2 → 4 AsBr3 + 3 SO2
Arsenic tribromide is a highly water soluble crystalline arsenic source for uses compatible with bromides and lower (acidic) pH. Most metal bromide compounds are water soluble for uses in water treatment, chemical analysis and in ultra high purity for certain crystal growth applications. Arsenic bromide is generally immediately available in most volumes.
It is soluble in hydrocarbons; carbon tetrachloride; very soluble in ether, benzene, chlorinated hydrocarbons, carbon disulfide, oils, and fats.
Chemical Reactivity
Arsenic tribromide is a Lewis acid, meaning it can accept a pair of electrons from a Lewis base. It reacts readily with water to form arsenic oxide and hydrogen bromide:
AsBr3 + 3 H2O → As2O3 + 6 HBr
Toxicity
Like many arsenic compounds, arsenic tribromide is highly toxic and poses significant health risks if ingested or inhaled. It can cause irritation to the skin, eyes, and respiratory tract.
Applications
Arsenic tribromide finds some limited use in organic synthesis, particularly in bromination reactions.
Handling
Due to its toxicity and reactivity, arsenic tribromide should be handled with extreme care in a well-ventilated area, preferably under a fume hood, and appropriate personal protective equipment should be worn.