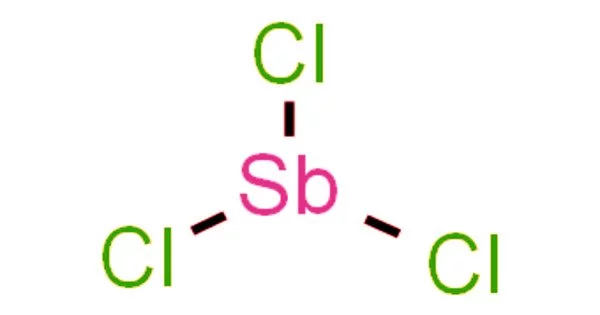
The chemical compound with the formula SbCl3 is antimony trichloride. Alchemists referred to it as butter of antimony because it is a soft colorless solid with a pungent odor. It is used as a reagent for detecting vitamin A and related carotenoids, forming a blue complex with the carotenoid that can be measured using colorimetry (the Carr-Price test). Antimony trichloride solutions were previously used to dissolve and remove horn stubs from calves and goats.
Properties
Antimony trichloride is a noncombustible, clear, colorless, crystalline solid. It is a colorless crystalline solid; orthorhombic crystal; hygroscopic; density 3.14 g/cm3; melts at 73.4°C; boils at 220.3°C; readily dissolves in water undergoing hydrolysis; soluble in dilute hydrochloric acid, ethanol, acetone, benzene, dioxane, and CS2.
- Chemical formula: Cl3Sb
- Molar mass: 228.11 g·mol−1
- Appearance: Colorless solid, very hygroscopic
- Odor: Sharp, pungent
- Density: 3.14 g/cm3 (25 °C); 2.51 g/cm3 (150 °C)
- Melting point: 73.4 °C (164.1 °F; 346.5 K)
- Boiling point: 223.5 °C (434.3 °F; 496.6 K)
- Solubility in water: 601.1 g/100 ml (0 °C); 1.357 kg/100 mL (40 °C)
- Solubility: Soluble in acetone, ethanol, CH2Cl2, phenyls, ether, dioxane, CS2, CCl4, CHCl3, cyclohexane, selenium(IV) oxychloride
- Insoluble in pyridine, quinoline, organic bases
Preparation
Antimony trichloride is prepared by reaction of chlorine with antimony, antimony tribromide, antimony trioxide, or antimony trisulfide. It also may be made by treating antimony trioxide with concentrated hydrochloric acid.

Reactions
SbCl3 is readily hydrolyzed and samples of SbCl3 must be protected from moisture. With a limited amount of water it forms antimony oxychloride releasing hydrogen chloride:
SbCl3 + H2O → SbOCl + 2 HCl
With more water, it forms Sb4O5Cl2 which on heating to 460° under argon converts to Sb8O44Cl12.
SbCl3 readily forms complexes with halides, but the stoichiometries are not a good guide to the composition, for example, the (C5H5NH)SbCl4 contains a chain anion with distorted SbIII octahedra. Similarly the salt (C4H9NH3)2SbCl5 contains a polymeric anion of composition [SbCl2−5]n with distorted octahedral SbIII.
SbCl3 is only a weak Lewis base, however, some complexes are known for example the carbonyl complexes, Fe(CO)3(SbCl3)2 and Ni(CO)3SbCl3.
Structure
SbCl3 has a pyramidal structure in the gas phase, with a Cl-Sb-Cl angle of 97.2° and a bond length of 233 pm. Each Sb in SbCl3 has three Cl atoms at 234 pm, demonstrating the persistence of the molecular SbCl3 unit; however, there are five neighboring Cl atoms, two at 346 pm, one at 361 pm, and two at 374 pm. These eight atoms can be thought of as forming a bicapped trigonal prism. These distances contrast with BiCl3, which has three close neighbors at 250 pm, two at 324 pm, and three at a mean of 336 pm. The point to note here is that all eight close neighbors of Bi are closer than the eight closest neighbors of Sb, demonstrating the tendency for Bi to adopt higher coordination numbers.
Uses
SbCl3 is a reagent used in the Carr-Price test to detect vitamin A and related carotenoids. Antimony trichloride reacts with the carotenoid to produce a blue complex that can be measured using colorimetry. Antimony trichloride has also been used as an adulterant in absinthe to enhance the louche effect. It has previously been used to dissolve and remove horn stubs from calves without the need to cut them off.
It is also used as a mordant and as a catalyst in polymerization, hydrocracking, and chlorination reactions, as well as in the production of other antimony salts. Its solution is used in the analysis of chloral, aromatics, and vitamin A. It could be used as a Lewis acid catalyst in synthetic organic transformations.
Although a solution of antimony trichloride in liquid hydrogen sulfide is a good conductor, its applications are limited due to the extremely low temperature or high pressure required for hydrogen sulfide to be liquid.