Bonding in Ionic Crystals and their Characteristic
Depending on the forces that hold the atoms, molecules or ions together in crystal lattice crystals are classified into four main types.
These are: (i) Ionic crystal, (ii) Molecular crystal, (iii) Network covalent crystal and (iv) Metallic crystal. Here briefly focus on Ionic crystal.
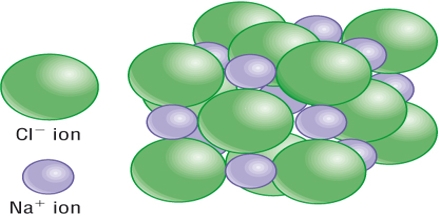
Ionic crystal: The ionic crystals are made up of positive and negative ions held together by electrostatic forces of attractions. An ionic crystal consists of ions bound together by electrostatic attraction. The bonds between the oppositely charged ions are ionic bonds. The arrangement of ions in a regular, geometric structure is called a crystal lattice. The important features of ionic crystals are:
(a) The ionic forces are non-directional.
(b) Since the electrostatic forces between ions are strong the ions are closely packed in ionic crystals and it is difficult to remove the ions from their respective positions in the lattice. As a result ionic crystals are very hard and have high melting points; like; NaCl- 8000C; KCl- 7900C.
(c) Ionic crystals are good conductors of heat and electricity either in molten state or in solution.
(d) Ionic crystals are soluble in polar solvents.
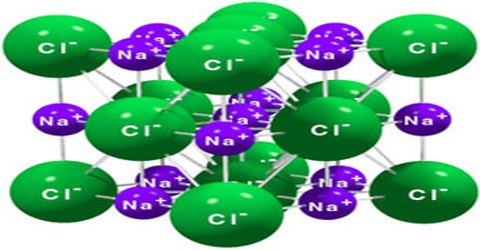
Sodium chloride is an ionic crystal in which each Na+ ion is surrounded by six negatively charged Cl– ions and vice versa (figure).