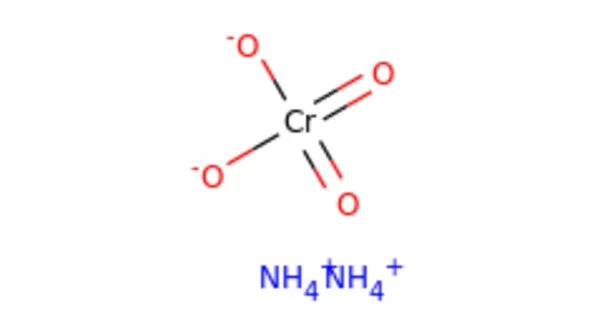
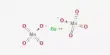
Ammonium chromate is a salt with the formula (NH4)2CrO4. It’s a hexavalent chromium chemical compound. It is a yellow, monoclinic crystal formed by ammonium hydroxide and ammonium dichromate; it is used in photography as a sensitizer for gelatin coatings. Hexavalent chromium refers to chemical compounds containing the element chromium in the +6 oxidation state. Because of its increased ability to enter cells and higher redox potential, chromium(VI) is more toxic than other oxidation states of the chromium atom.
It is used to prevent corrosion, as well as in dyeing, photography, and a variety of chemical reactions. It is a fungicide and a fire retardant. It is frequently used in photography, textile printing, and the application of chromate dyes to wool. It is also employed as an analytical reagent, a catalyst, and a corrosion inhibitor. It is water soluble and, when applied, can cause irritation in the mucous membranes, eyes, respiratory tract, skin, and other areas. After prolonged contact, it may cause skin sensitization. It is also known to be carcinogenic (causes cancer), and it can cause tissue ulceration as well as liver and kidney damage.
Properties
It is a yellow monoclinic crystal. The relative density was 1. 91. Decomposition was initiated by heating to 180 °c. It is soluble in cold water, slightly soluble in ammonia, acetone, insoluble in alcohol. Long-term storage can be decomposed to release ammonia, partial conversion to ammonium dichromate.
- Chemical formula: (NH4)2CrO4
- Molar mass: 152.07 g/mol
- Appearance: yellow crystals
- Density: 1.90 g/ml
- Melting point: 185 °C (365 °F; 458 K) decomposes
- Solubility in water: 24.8 g/100ml (0 °C)

Preparation Method
Neutralization method: Ammonium chromate solution is made by adding aqueous ammonia to a reactor containing ammonium dichromate to perform a neutralization reaction, which is then followed by evaporation and concentration, cooling crystallization, solid-liquid separation, and drying to yield the final ammonium chromate product.
Double decomposition method: Potassium chromate is added to a reactor containing water, and ammonium sulfate solution is slowly added while stirring to generate ammonium chromate and potassium sulfate solution, which is filtered to remove potassium sulfate and concentrated by evaporation, cooling crystallization, solid-liquid separation, drying, and prepared ammonium chromate products.
Uses
In photography, ammonium chromate is used as a sensitizer for gelatin coatings. It is frequently used in photography, textile printing, and the fixation of chromate dyes on wool. It is also used as a catalyst and an analytical reagent.