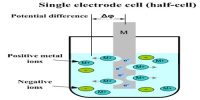
Half-Cells
A half cell is one of the two electrodes in a galvanic cell or simple battery. For example, in the Zn-Cu battery, the two half cells make an oxidizing-reducing couple.
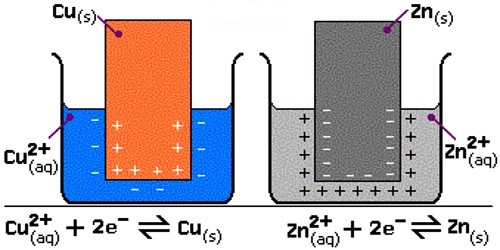
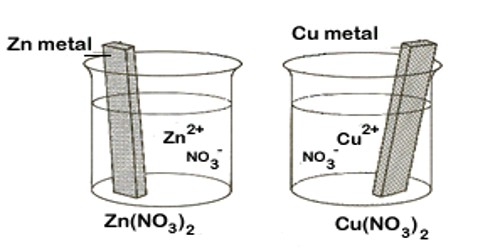
The reaction taking place in an electrochemical cell may be split up into two reactions at the two electrodes. Similarly, the cell may be split up into two half-cells, one at each electrode; an electrode dipping into a solution is said to constitute a half-cell. Thus in the Daniel cell Zn │ Zn2+ and Cu │ Cu2+ constitute two half-cells.
In a Zn-Cu battery, when two half cells are connected, the following reaction takes place:
Reduction reaction takes place at the cathode:
Cu2+ + e → Cu; (Cu2+ is the oxidizing agent and Cu the reducing agent.)
Oxidation reaction takes place at the anode:
Zn → Zn2+ + 2 e– (Zn is the reducing agent, and Zn2+ the oxidizing agent.)
It is convenient to describe the electrochemical processes in terms of half-cells, as two half-cells can be suitably arranged to produce a desired reaction in a cell. Various types of reaction may be made to occur electrochemically and various electrodes or half-cells may be used. Some of these are: Metal-metal ion electrode, Amalgam electrode etc.
A half-cell is half of an electrolytic or voltaic cell, where also oxidation or decline occurs. The half-cell reaction at the anode is oxidation, while the half-cell reaction at the cathode is reduction.