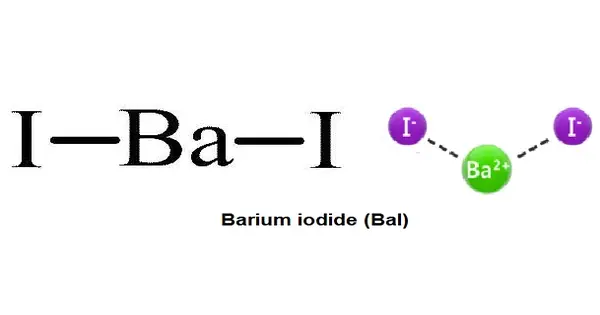
Barium iodide is an inorganic compound with the formula BaI2. It is a chemical compound composed of barium and iodine. The compound exists as an anhydrous and a hydrate (BaI2(H2O)2), both of which are white solids. When heated, hydrated barium iodide converts to anhydrous salt. The hydrated form is freely soluble in water, ethanol, and acetone.
Barium iodide typically exists as a white crystalline solid at room temperature. It is soluble in water, alcohol, and acetone. The molar mass is approximately 391.13 g/mol. It has a relatively high melting point, around 725°C (1,337°F). The compound decomposes before reaching a boiling point under normal atmospheric pressure. Its density varies depending on the form but generally falls around 4.9 g/cm³.
Properties
- Chemical formula: BaI2 (anhydrous); BaI2·2H2O (dihydrate)
- Molar mass: 391.136 g/mol (anhydrous); 427.167 g/mol (dihydrate)
- Appearance: White orthorhombic crystals (anhydrous) colorless crystals (dihydrate)
- Odor: odorless
- Density: 5.15 g/cm3 (anhydrous); 4.916 g/cm3 (dihydrate)
- Melting point: 711 °C (1,312 °F; 984 K) (anhydrous); decomposes at 740 °C (dihydrate)
- Solubility in water: 166.7 g/100 mL (0 °C); 221 g/100 mL (20 °C); 246.6 g/100 mL (70 °C)
- Solubility: soluble in ethanol, acetone
Structure
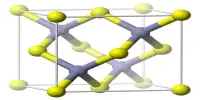
The structure of the anhydrous form resembles that of lead(II) chloride with each Ba center bound to nine iodide ligands and has a crystalline packing structure that is quite similar to BaCl2. It is a source of iodide ions and can be used in various chemical reactions where iodide ions are needed. Barium iodide reacts with other substances to form insoluble barium compounds. It tends to absorb moisture from the air, so it should be stored in a dry environment to prevent degradation or clumping.
Reactions
Anhydrous BaI2 can be prepared by treating Ba metal with 1,2-diiodoethane in ether.
BaI2 reacts with alkyl potassium compounds to form organobarium compounds.
BaI2 can be reduced with lithium biphenyl, to give a highly active form of barium metal.
Uses
Barium iodide has applications in organic synthesis, particularly in the preparation of other iodine-containing compounds. It is also used in some types of chemical analysis and in certain industries as a source of iodine.
Toxicity
Like other barium compounds, barium iodide is toxic and should be handled with care. Ingestion or inhalation of significant amounts can lead to health issues. It may release toxic fumes when heated to decomposition, including iodine and barium oxide.