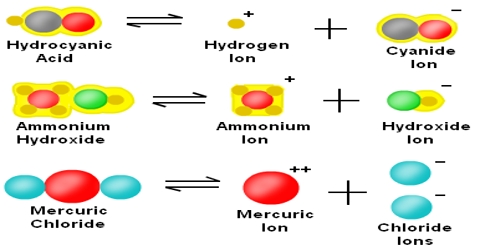
Molecular dissociation
Upon heating, the density of aluminum chloride vapour decreases and at 800°C the density corresponds to the formula Aluminium chloride (AlCl3), i.e., one molecule of Al2Cl6 has broken down to form 2 molecules of AlCl3. This phenomenon in which a molecule breaks down to form two or more molecules is known as dissociation. As the dissociation here is caused by heat the phenomenon is called thermal dissociation, to distinguish it from electrolytic dissociation.
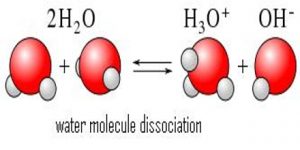
Dissociation is a general process in which molecules separate or split into smaller particles such as atoms, ions or radicals, usually in a reversible manner. Dissociation is not the same as decomposition. In thermal decomposition the molecular species formed from breakdown do not combine upon cooling, but in dissociation the separated pans recombine upon cooling provided these are not removed from the vessel in which they are heated. Basic copper carbonate (CuCO3) yields, Copper oxide (CuO) and CO2 upon heating. This is a case of decomposition as CuO and CO2 will not recombine to form CuCO3 upon cooling. When AlCl3 vapour heated to 8000C is cooled to 200°C or below Al2Cl6 is formed again as shown by density measurements.