Hydrogen telluride is an inorganic chemical having the formula H2Te. It is a colourless gas that is a hydrogen chalcogenide and the simplest hydride of tellurium. Although unstable in ambient air, the gas can survive at very low concentrations long enough to be detected by the stench of decaying garlic at extremely low concentrations, or by the unpleasant odour of rotten leeks at slightly greater quantities.
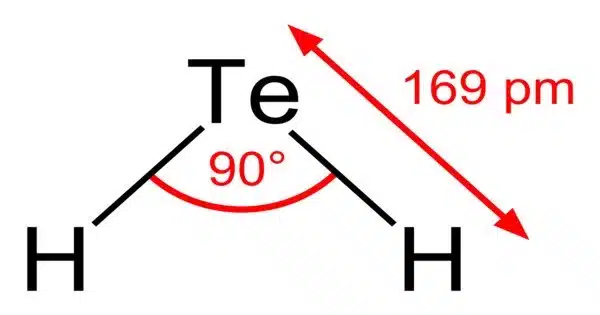
Most Te-H bonds (tellurols) are unstable in terms of H2 leakage. H2Te is chemically and physically identical to hydrogen selenide, and both are acidic. The H-Te-H angle is roughly 90°. Volatile tellurium compounds frequently emit unpleasant scents reminiscent of rotting leeks or garlic.
Properties
Hydrogen telluride is a chemical compound composed of hydrogen and tellurium. Its chemical formula is H2Te. It is a colorless, highly toxic, and flammable gas with a foul odor similar to rotten garlic or sulfur.
- Chemical formula: H2Te
- Molar mass: 129.6158 g mol−1
- Appearance: colourless gas
- Odor: Pungent, resembles rotting garlic or leeks
- Density: 3.310 g/L, gas; 2.57 g/cm3 (−20 °C, liquid)
- Melting point: −49 °C (−56 °F; 224 K)
- Boiling point: −2.2 °C (28.0 °F; 270.9 K) (unstable above −2 °C)
- Solubility in water: 0.70 g/100 mL
- Acidity (pKa): 2.6
- Conjugate acid: Telluronium
- Conjugate base: Telluride
Reactions
Hydrogen telluride is a hydride of tellurium and belongs to the class of binary hydrogen compounds. It is formed when tellurium reacts with hydrogen or when certain tellurium-containing minerals are exposed to acidic environments.
H2Te is an endothermic compound, degrading to the elements at room temperature:
H2Te → H2 + Te
Light accelerates the decomposition. It is unstable in air, being oxidized to water and elemental tellurium:
2 H2Te + O2 → 2 H2O + 2 Te
It is almost as acidic as phosphoric acid (Ka = 8.1×10−3), having a Ka value of about 2.3×10−3. It reacts with many metals to form tellurides.
Health hazards
Hydrogen telluride is dangerous due to its toxicity and foul odour and should be handled with extreme caution. Even little amounts of this gas can cause major health problems, and long-term exposure can be lethal. As a result, working with hydrogen telluride in a well-ventilated room and with suitable safety equipment is critical.