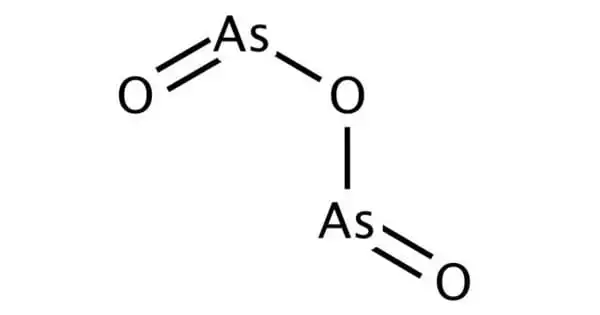
Arsenic trioxide is a medicine and an inorganic chemical. It is the most important commercial arsenic compound and the primary starting material for arsenic chemistry. As an industrial chemical, its main applications include the production of wood preservatives, insecticides, and glass. It is an extremely hazardous byproduct of certain ore processing, such as gold mining. It is used to treat a kind of cancer known as acute promyelocytic leukemia as a medicine. It is injected into a vein for this purpose.
Vomiting, diarrhea, edema, shortness of breath, and headaches are common adverse effects. APL differentiation syndrome and heart difficulties are possible severe side effects. Use during pregnancy or while breastfeeding may be harmful to the baby. As2O3 is the formula for arsenic trioxide. Its mechanism in treating cancer is not entirely clear.
Properties
Arsenic Trioxide is a small-molecule arsenic compound with antineoplastic activity. It appears as white or transparent, glassy amorphous lumps or crystalline powder. It is slightly soluble in water, but dissolves very slowly; more soluble in hot water.
- Formula: As2O3
- Molar mass: 197.840 g·mol−1
- Density: 3.74 g/cm3
- Melting point: 312.2 °C (594.0 °F)
- Boiling point: 465 °C (869 °F)
- Solubility in water: 20 g/L (25 °C)

Uses
- Medical
Arsenic trioxide is used to treat acute promyelocytic leukemia, a kind of malignancy (APL). It can be used in cases when previous treatments, such as all-trans retinoic acid (ATRA), have failed or as part of the first therapy of newly diagnosed patients. This initial treatment may comprise a combination of arsenic trioxide and all-trans retinoic acid therapy (ATRA).
- Manufacturing
Industrial applications include utilization as a precursor to forestry products, the manufacturing of colorless glass, and electronics. As the primary arsenic component, trioxide is the precursor to elemental arsenic, arsenic alloys, and arsenide semiconductors. The trioxide is converted into the bulk arsenic-based chemicals sodium arsenite and sodium cacodylate.
Production and occurrence
Arsenic trioxide can be generated via routine processing of arsenic compounds including the oxidation (combustion) of arsenic and arsenic-containing minerals in air. Illustrative is the roasting of orpiment, a typical arsenic sulfide ore.
2 As2S3 + 9 O2 → 2 As2O3 + 6 SO2
However, the majority of arsenic oxide is derived as a volatile by-product of the processing of other ores. Arsenopyrite, a frequent contaminant in gold and copper ores, for example, releases arsenic trioxide when heated in air. The processing of such minerals has resulted in numerous poisonings. Only in China are arsenic ores mined on purpose.
In the laboratory, it is prepared by hydrolysis of arsenic trichloride:
2 AsCl3 + 3 H2O → As2O3 + 6 HCl
As2O3 occurs naturally as two minerals, arsenolite (cubic) and claudetite (monoclinic). Both are relatively rare secondary minerals found in oxidation zones of As-rich ore deposits.
Reactions
Arsenic trioxide is an amphoteric oxide with weakly acidic aqueous solutions. As a result, it dissolves easily in alkaline solutions to form arsenites. Although it will dissolve in hydrochloric acid, it is less soluble in acids.
With anhydrous HF and HCl, it gives AsF3 and the trichloride:
As2O3 + 6 HX → 2 AsX3 + 3 H2O (X = F, Cl)
Only with strong oxidizing agents such as ozone, hydrogen peroxide, and nitric acid does it yield arsenic pentoxide, As2O5 or its corresponding acid:
2 HNO3 + As2O3 + 2 H2O → 2 H3AsO4 + N2O3
In terms of its resistance to oxidation, arsenic trioxide differs from phosphorus trioxide, which readily combusts to phosphorus pentoxide.
Reduction gives elemental arsenic or arsine (AsH3) depending on conditions:
As2O3 + 6 Zn + 12 HNO3 → 2 AsH3 + 6 Zn(NO3)2 + 3 H2O
This reaction is used in the Marsh test.