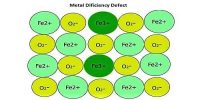
Amorphous solids possess properties of incompressibility and rigidity to a certain extent but they do not have definite geometrical forms.
When certain liquids are cooled rapidly there is no formation of crystals at a definite temperature, such as occurring on slow cooling. The viscosity of the liquid increases steadily and finally a glassy substance is formed.
The chief characteristics of a glass are hardness, rigidity and ability to withstand shearing stresses which are all properties of the solid state. On the other hand glasses are optically isotropic and on heating without any sharp transition passes into a mobile liquid. At a high temperature glasses undergo phase transition when crystals separate first as they do form supercooled liquid. Therefore, glasses are regarded as amorphous solids or super cooled liquids as well. Thus, glassy or vitreous state is a condition in which certain substance can exist, lying between the solid and liquid states.