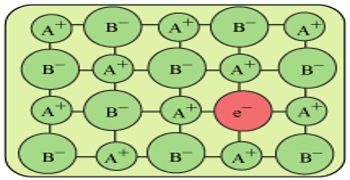
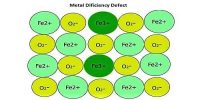
If a crystal of NaCl is heated in sodium vapour, it acquires a yellow colour. This yellow colour is due to the formation of a non-stoichiometric compound of NaCl in which there is a slight excess of sodium ions. This defect is called the metal excess defect.