Aluminoxanes are a class of inorganic compounds that contain both aluminum and oxygen atoms, typically in the form of a polymeric structure. They are commonly used as cocatalysts in the production of polyolefins, which are widely used in plastics, coatings, and other industrial applications.
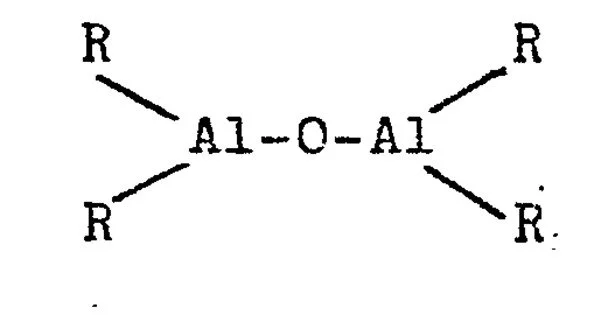
Aluminoxanes are organoaluminium compounds with the formula [RAlO]m[R2AlO0.5]n[R2AlOH]o, where R = organic substituent. The following structural rules apply: Al is tetrahedral and O is three-coordinate.
Properties
- Stability: stable in air and water, and are relatively unreactive towards other chemicals.
- Solubility: generally soluble in organic solvents such as toluene, hexane, and chloroform.
- Acidic properties: Lewis acids, which means they can act as electron pair acceptors in chemical reactions.
- Thermal stability: generally stable at high temperatures, which makes them useful in high-temperature applications.
Aluminoxanes are usually synthesized by the reaction of an aluminum alkyl or aluminum alkoxide with water or an alcohol, typically in the presence of an acid catalyst. The resulting product is a complex mixture of oligomeric and polymeric species with a range of chain lengths and structures.
Aluminoxanes have been extensively studied as cocatalysts in the Ziegler-Natta polymerization of olefins, which is a widely used industrial process for the production of polyethylene and polypropylene. They help to activate the catalyst and increase its activity, as well as controlling the molecular weight and other properties of the resulting polymers.
Application
Aluminoxanes are widely used as co-catalysts in olefin polymerization reactions, where they enhance the activity and selectivity of transition metal catalysts. They are commonly used as cocatalysts in the polymerization of olefins, especially in Ziegler-Natta polymerization of polyethylene and polypropylene.
Methylaluminoxane is widely used in alkene polymerization. Typically, these compounds are synthesized through the partial hydrolysis of trialkylaluminium compounds. Aluminoxanes are used to activate catalytic olefin polymerisation catalysts such as the Ziegler-Natta catalyst. They also act as a scavenger for impurities (for example, water) in reactions that are sensitive to these impurities. As solutions, aluminoxane is encountered as white solids.
In addition to their use in polymerization, aluminoxanes have other applications. They can be used as catalysts for organic reactions, such as the Friedel-Crafts reaction and the Diels-Alder reaction. They also have potential applications in the field of heterogeneous catalysis, especially in the oxidation of hydrocarbons. Overall, aluminoxanes play an important role in industrial chemistry and are crucial for the production of many materials that we use every day.