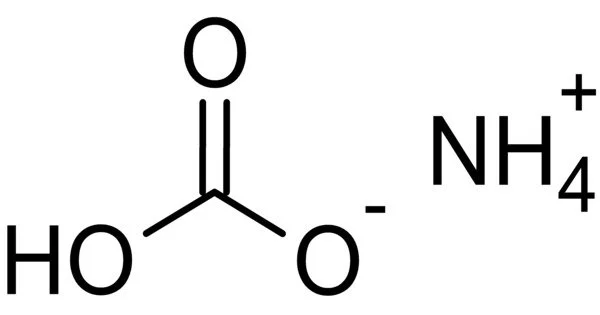
The inorganic compound ammonium bicarbonate has the formula (NH4)HCO3. It appears as a white crystalline solid with an ammonia-like odor. The compound has several names that reflect its long history. It is the bicarbonate salt of the ammonium ion in chemistry. It’s a colorless solid that easily degrades into carbon dioxide, water, and ammonia.
It dissolves in water to form a slightly alkaline solution. Furthermore, it is insoluble in a wide range of organic solvents. It is harmful to the environment, and immediate action should be taken to prevent its spread. It is widely used in the food processing industry.
Properties
Ammonium hydrogen carbonate is a crystalline solid white in colour which smells like ammonia. It is soluble in water but insoluble in ethanol, acetone, alcohol, and benzene. It exists as a white crystalline solid that carries a density of 1.59 g/mL and a melting point of 41.9 °C. It has a strong aroma of ammonia, and it is highly soluble in water.
- Chemical formula: NH4HCO3
- Molar mass: 79.056 g/mol
- Density: 1.586 g/cm3
- Melting point: 41.9 °C (107.4 °F; 315.0 K) decomposes
- Solubility in water” 11.9 g/100 mL (0 °C); 24.8 g/100 mL (25 °C)
- Solubility: insoluble in methanol

Production
Ammonium bicarbonate is produced by combining carbon dioxide and ammonia:
CO2 + NH3 + H2O → (NH4)HCO3
Since ammonium bicarbonate is thermally unstable, the reaction solution is kept cold, which allows the precipitation of the product as white solid. About 100,000 tons were produced in this way in 1997.
Ammonia gas passed into a strong aqueous solution of the sesquicarbonate (a 2:1:1 mixture of (NH4)HCO3, (NH4)2CO3, and H2O) converts it into normal ammonium carbonate ((NH4)2CO3), which can be obtained in the crystalline condition from a solution prepared at about 30 °C. This compound on exposure to air gives off ammonia and reverts to ammonium bicarbonate.
Reactions
It dissolves in water to give a mildly alkaline solution. It is insoluble in acetone and alcohols.
Ammonium bicarbonate decomposes above about 36 °C into ammonia, carbon dioxide, and water in an endothermic process and so causes a drop in the temperature of the water:
NH4HCO3 → NH3 + H2O + CO2.
When treated with acids, ammonium salts are also produced:
NH4HCO3 + HCl → NH4Cl + CO2 + H2O.
Reaction with base produces ammonia.
It reacts with sulfates of alkaline-earth metals precipitating their carbonates:
CaSO4 + 2 NH4HCO3 → CaCO3 + (NH4)2SO4 + CO2 + H2O.
It also reacts with alkali metal halides, giving alkali metal bicarbonate and ammonium halide:
NH4HCO3 + NaCl → NH4Cl + NaHCO3;
NH4HCO3 + KI → NH4I + KHCO3;
NH4HCO3 + NaBr → NH4 r + NaHCO3.
Salt of hartshorn
Ammonium carbonate-containing compositions have long been known. They were once commercially produced and were known as sal volatile or salt of hartshorn. It was made by dry distilling nitrogenous organic matter such as hair, horn, and leather. This material also contains ammonium carbamate (NH4CO2NH2) and ammonium carbonate ((NH4)2CO3) in addition to ammonium bicarbonate. It is also known as ammonium sesquicarbonate. It has a strong ammoniacal odor, and when digested with alcohol, the carbamate dissolves, leaving an ammonium bicarbonate residue.
Uses
In the food industry, ammonium bicarbonate is used as a leavening agent for flat baked goods such as cookies and crackers. Prior to the availability of modern baking powder, it was widely used in the home.
Because of its leavening and stabilizing properties, as well as its acid regulating capacity, ammonium bicarbonate is a widely used ingredient in the bakery industry. Common applications of Ammonium Bicarbonate:
- Cookies
- Crackers
- Pastas
- Frozen dairy products
- Bakery ingredients
- Pigments and paints
- Agriculture
- Fire extinguishers
It is commonly used as a low-cost nitrogen fertilizer in China, but it is being phased out in favor of urea for quality and stability. This compound is used in the manufacture of fire-extinguishing compounds, pharmaceuticals, dyes, and pigments, as well as a basic fertilizer as an ammonia source. Ammonium bicarbonate is still widely used in the plastics and rubber industries, ceramic manufacturing, chrome leather tanning, and catalyst synthesis.
It is also used to slightly alkalinize solutions during chemical purification processes such as high-performance liquid chromatography. Because it completely decomposes to volatile compounds, it allows for the rapid recovery of the compound of interest via freeze-drying.
Safety
Ammonium bicarbonate is a skin, eye, and respiratory system irritant. Short-term health effects may occur immediately or shortly after ammonium bicarbonate exposure. Coughing, wheezing, and/or shortness of breath can result from inhaling ammonium bicarbonate, which can irritate the nose, throat, and lungs. Repeated exposure may result in bronchitis, with coughing and/or shortness of breath. Ammonium bicarbonate exposure can cause health effects that can last for months or years.
Operations should be enclosed whenever possible, and local exhaust ventilation at the site of chemical release is recommended. Respirators are required if no local exhaust ventilation or enclosure is used. Wear protective work clothing and immediately change and thoroughly wash your clothes after being exposed to ammonium bicarbonate.