Calcium alginate is a water-insoluble, gelatinous, cream-colored substance formed by combining aqueous calcium chloride and aqueous sodium alginate. Calcium alginate is also used in plant tissue culture for enzyme entrapment and the formation of artificial seeds. Alginate is the most commonly used polysaccharide in wound healing.
Alginate is a broad term that refers to a group of polysaccharides produced by seaweed, brown algae, and bacteria. “Alginate” usually refers to alginic acid salts, but it can also refer to alginic acid derivatives and alginic acid itself; in some publications, the term “algin” is used instead of alginate. Alginate is found in brown algae cell walls as the calcium, magnesium, and sodium salts of alginic acid.
Properties
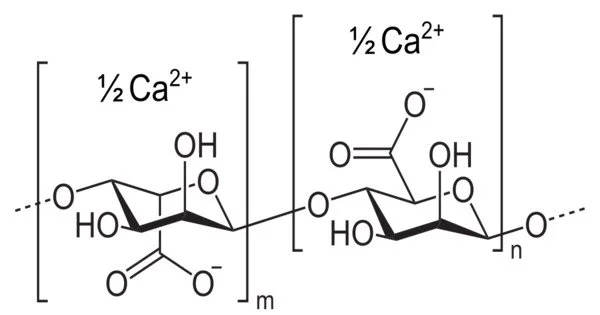
- Chemical formula: (C12H14CaO12)n
- Appearance: Solid
- Specific Gravity: 1.6
- Color: White to Gray to Brown
- Density: 2.1173 g/cm3
Structure
The structure of calcium alginate hydrogels has long been a subject of scientific investigation due to their technological importance. The “Egg-Box” model, proposed in the 1970s, is the most well-known theory of alginate-metal binding. This model is based on the distinct arrangement of hydroxyl groups in polymeric α-L-guluronate (G) units, which provide cavities for cations to sit in, similar to eggs in an egg box. While the reality is likely to be much more complicated, the Egg-Box model is a useful explanatory tool, with over 1500 citations to the original paper proposing the model.
Preparation
- Extraction of alginate
To extract the alginate, the seaweed is broken into pieces and mixed with a hot solution of an alkali, usually sodium carbonate. Over the course of about two hours, the alginate dissolves as sodium alginate, yielding a very thick slurry. This slurry also contains the part of the seaweed that does not dissolve, primarily cellulose. This insoluble residue must be removed from the solution. The solution is too thick (viscous) to filter and must be diluted with a large amount of water. After dilution, the solution is forced through a filter cloth in a filter press.
The undissolved residue, on the other hand, is very fine and can quickly clog the filter cloth. As a result, before beginning filtration, a filter aid, such as diatomaceous earth, must be added; this keeps most of the fine particles away from the filter cloth’s surface and facilitates filtration. However, filter aid is expensive and can add significantly to costs. Some processors force air into the extract as it is diluted with water to reduce the amount of filter aid required. Fine air bubbles attach themselves to the residue particles.
- Preparation of calcium alginate from sodium alginate
A calcium salt, such as calcium chloride, can be added to a sodium alginate solution to produce calcium alginate. This results in the formation of insoluble calcium alginate salt, which precipitates out of solution. The calcium alginate can then be redissolved in various sodium carbonate solutions to produce alginate products with different sodium-to-calcium ratios. This has an effect on the physical and chemical properties of alginate.
Uses
Uses of calcium alginate are:
- in plant tissue culture to produce insoluble artificial seeds
- for immobilizing enzymes by entrapment
- to produce an edible substance
- incorporated into wound dressings (alginate dressings) as a hemostatic
- as an alginate hydrogel, can be used for a controlled-release drug delivery system
- used in molecular gastronomy in spherification.