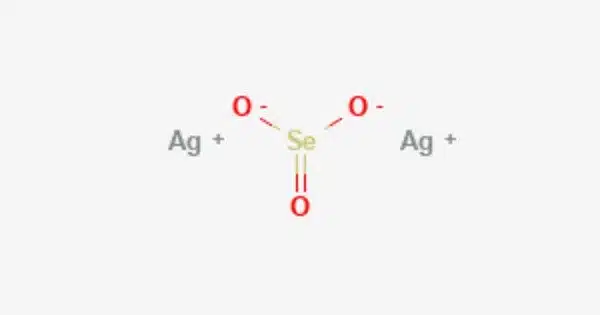
Silver selenite is generated during the recovery of selenium from copper anode slimes by oxidative roasting, which causes some silver selenide to be transformed to selenite. It can also be made through a precipitation process between silver nitrate and sodium selenite:
Silver is a good conductor of electricity, has a high thermal conductivity, and is widely employed in a variety of industrial applications, including electronics and photography. It is commonly used in jewelry, coinage, and silverware. It also possesses antimicrobial characteristics, making it valuable in medical applications and water purification.
Another method is the reaction between selenium and silver nitrate:
3 Se + 6 AgNO3 + 3 H2O → 2 Ag2Se + Ag2SeO3 + 6 HNO3
Properties
Selenite refers to a variety of the mineral gypsum, which is a hydrated calcium sulfate. The name “selenite” comes from the Greek word for moon, likely due to its pearly and moon-like luster. It typically forms as transparent, colorless crystals with a striated or fibrous structure. It is a soft mineral and can be scratched easily.
- Chemical formula: Ag2SeO3
- Molar mass: 342.69 g/mol
- Appearance: crystalline needles
- Density: 5.930 g/cm3
- Melting point: 530 °C (986 °F; 803 K)
- Boiling point: decomposes above 550 °C (1,022 °F; 823 K)
- Solubility in water: slightly soluble
- Solubility: soluble in acids
Selenite is a crystalline form of the mineral gypsum, typically colorless, transparent, and composed of calcium sulfate. It is a popular mineral in the metaphysical and spiritual community due to its purported healing properties and its association with clarity and high vibrations. On the other hand, silver is a chemical element with the symbol Ag. It is a transition metal known for its lustrous appearance and conductivity. Silver itself is not commonly associated with metaphysical properties like crystals.