Indeed, light can play an important role in influencing the structural conversion of chiral compounds. This effect is known as photoisomerization, and it is especially important in chemistry. Enantiomers are compounds that exist in two non-superimposable mirror-image forms known as chiral molecules. These enantiomers can have a wide range of chemical and biological properties.
A group of chemists has devised a revolutionary notion that converts a mixture of molecules that behave like mirror images into a single form. They employ light as an external energy source to accomplish this. The conversion is important for drug production, for example.
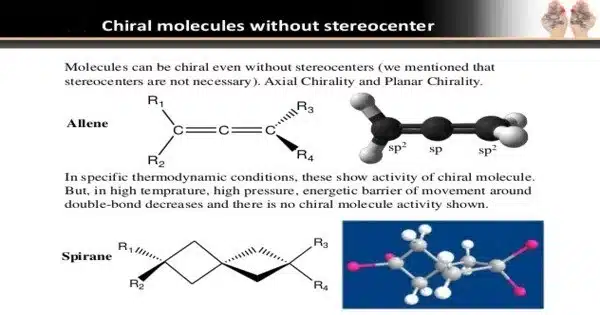
Certain organic molecules, like human hands, interact with each other like an image and its reflection, a phenomenon known to chemists as “chirality” or “handedness.” Both enantiomers, or mirror versions of the same molecule, often have different biological properties. For example, in drug discovery, only one of the structures is frequently relevant. Chemical synthesis methods, on the other hand, frequently produce a 1:1 combination of both types. As a result, the selective conversion of complex combinations into a single selected form is critical.
The researchers use a light-activated aluminium complex as a catalyst to selectively transform a mixture of molecules that behave like mirror images to a single form. The reaction process was studied experimentally as well as computationally.
Prof. Ryan Gilmour and Prof. Johannes Neugebauer led a team of researchers from the Institute of Organic Chemistry and the Center for Multiscale Theory and Computation at the University of Münster in developing a novel concept in which this conversion is enabled by light as an external energy source. The findings have been published in the journal Nature.
The researchers use a light-activated aluminum complex as a catalyst to selectively transform a mixture of molecules that behave like mirror images to a single form. The reaction process was studied experimentally as well as computationally.
Photoisomerization involves the reversible transformation of a molecule from one isomeric form to another upon absorption of light. This transformation can occur in both achiral and chiral molecules, but in the context of chiral molecules, it’s of particular interest because it can lead to changes in their optical activity, which is a measure of their ability to rotate plane-polarized light.
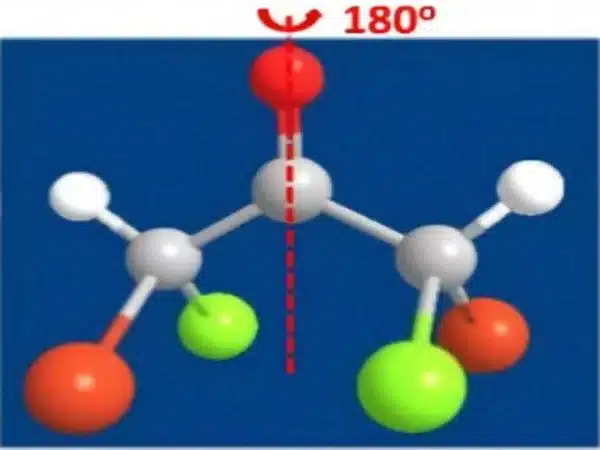
The extensive computer-based analysis greatly aided comprehension of the underlying mechanisms. Because the aluminum complex utilized is a common catalyst for chemical processes driven by heat, the new paradigm impresses with its operational simplicity and broad applicability. Translation to light-mediated processes is now being considered in order to enable a multitude of novel reactivities with high spatial control.
One of the major issues in modern organic chemistry is achieving spatial control in light-mediated reactions. Typically, two separate catalysts are used in a single reaction: a photocatalyst, which initiates the reactivity, and a second catalyst, which controls the spatial arrangement of the molecules.
To date, however, only the insertion of customized recognition motifs in the catalyst and substrate structures has resulted in the successful integration of both functionalities in a single catalyst structure. The groups offer a catalyst that modulates reactivity and selectivity at the same time in this study. It attaches to simple ketones, a functional group found in organic compounds, eliminating the need for customized components. Furthermore, the catalyst is made of plentiful aluminum, which is less expensive than the transition metals often found in photocatalysts.