LIBs (lithium-ion batteries) are used to power electric vehicles and devices. With the prevalence of these expected to rise, efforts have been made to improve the performance and longevity of LIBs. Researchers from Japan’s Advanced Institute of Science and Technology have demonstrated that adding a specific polymer composite binder to the silicon anode of LIBs can significantly improve structural stability, making it viable for much more powerful, long-lasting LIBs and changing the future of the technologies it drives.
When you think of a battery, you probably think of lithium-ion. Lithium-ion batteries (LIBs) have become the dominant type of battery in both low-power consumer electronic devices such as mobile phones and high-power applications such as electric vehicles and energy storage due to their light weight, high energy density, and ability to deliver three times as much current as other types of rechargeable batteries.
A typical lithium-ion battery nowadays consists of a positive electrode (cathode) made of a lithium-containing substance, a negative electrode (anode) composed of graphite, and electrolyte — the layer between the electrodes through which ions travel. When a battery is charged, lithium ions travel from the cathode to the anode, where they are stored. During the discharge process, the lithium is ionized and returns to the cathode.
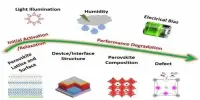
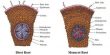
Recently, there has been increased interest in adopting silicon as the anode material because it is more available and thus less expensive, and it has a larger theoretical discharge capacity than graphite. It does, however, have a significant drawback: repetitive charging and discharging causes the silicon particles to enlarge and burst. As a result, a thick solid-electrolyte interface (SEI) forms between the electrolyte and the anode, impeding the flow of lithium ions between the electrodes.
The design and implementation of innovative polymer composites containing n-type conducting polymers (CPs) and proton donating polymers with hydrogen bonded networks, such as P-BIAN/PAA, have a promising future in high-capacity electrode materials.
Prof. Matsumi
To improve the performance of silicon anodes in LIBs, a team from Japan Advanced Institute of Science and Technology (JAIST) led by Professor Noriyoshi Matsumi and including Dr. Agman Gupta and Senior Lecturer Rajashekar Badam developed a binder for the silicon particles that can improve their stability and maintain a thin SEI layer. A thin SEI layer, as opposed to a thick one, is advantageous because it prevents the anode and electrolyte from spontaneously reacting with each other. The study’s findings were published in the journal ACS Applied Energy Materials.
The binder is a polymer composite made up of an n-type conducting polymer poly(bisiminoacenaphthenequinone) (P-BIAN) and a carboxylate-containing polymer poly(acrylic acid) (PAA), which are joined by hydrogen bonds. The composite polymer structure acts like a net, holding the silicon particles together and preventing them from rupturing. The hydrogen bonds between the two polymers allow the structure to self-repair because the polymers can reconnect themselves at any time. Furthermore, P-n-doping BIAN’s ability improves anode conductivity and maintains a thin SEI by reducing electrolytic breakdown of the electrolyte on the anode.
The binder was tested using an anodic half-cell made of silicon nanoparticles with graphite (Si/C), the binder (P-BIAN/PAA), and an acetylene black (AB) conductive additive. A charge-discharge cycle was performed on the Si/C/(P-BIAN/PAA)/AB anode. For over 600 cycles, the P-BIAN/PAA binder was shown to stabilize the silicon anode and sustain a specific discharge capacity of 2100 mAh g-1. The capacity of the bare silicon-carbon anode, on the other hand, decreased to 600 mAh g-1 after 90 cycles.
Following the test, the researchers removed the anode and analyzed the material for cracks caused by silicon breakage. A spectroscopic and microscopic study after 400 cycles revealed a smooth structure with just a few microcracks, showing that the addition of the binder was able to improve the structural integrity of the electrode and maintain a homogeneous SEI.
The results show that adding the binder can improve the properties of the silicon anode and make it practical. “The design and implementation of innovative polymer composites containing n-type conducting polymers (CPs) and proton donating polymers with hydrogen bonded networks, such as P-BIAN/PAA, have a promising future in high-capacity electrode materials,” Prof. Matsumi explains.
As the demand for lithium-ion batteries grows, silicon, the eighth-most abundant element on the planet, will be a promising environmentally benign alternative to graphite. The application of binders improves its structural stability and conductivity, making it more appropriate for use in future lithium-ion batteries. “This composite binder design method will enable greater adoption of EVs, the development of alternative battery-powered vehicles, and the manufacturing of drones, which require a higher energy density for advanced performance,” Prof. Matsumi explains.