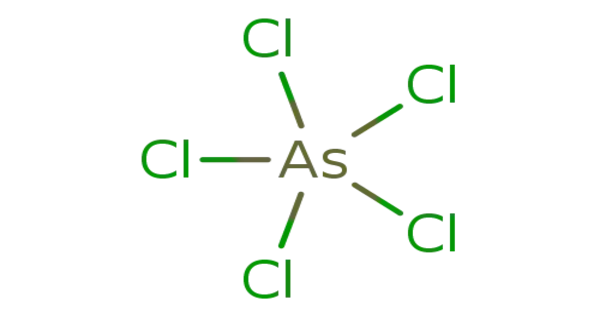
Arsenic pentachloride is a chemical compound that contains arsenic and chlorine. In 1976, this chemical was created by irradiating arsenic trichloride (AsCl3) with UV light in liquid chlorine at -105 °C. AsCl5 decomposes at around -50°C. The solid structure was ultimately determined in 2001. AsCl5 has a trigonal bipyramidal structure comparable to phosphorus pentachloride (PCl5), with shorter equatorial bonds than axial bonds (As-Cleq = 210.6 pm, 211.9 pm; As-Clax = 220.7 pm).
It dissolves in nonpolar solvents such as carbon tetrachloride and chloroform. However, it decomposes slowly in water, producing arsenic oxychlorides and hydrogen chloride. It is very reactive and can function as a Lewis acid, readily absorbing lone pairs of electrons. It reacts violently with water to produce hydrogen chloride and arsenic oxychlorides.
Properties
- Physical State: It is a colorless to pale yellow liquid at room temperature. It has a pungent odor.
- Melting and Boiling Point: It has a melting point of around -1.1°C and a boiling point of around 130°C. However, it tends to decompose before reaching its boiling point.
- Stability: While it is stable under certain conditions, it can decompose over time, especially when exposed to moisture or high temperatures.
- Molar mass: 252.1866 g/mol
Preparation
Arsenic pentachloride can be made by either directly chlorinating arsenic or reacting arsenic trichloride with chlorine gas.
The pentachlorides of the elements above and below arsenic in group 15, phosphorus pentachloride and antimony pentachloride, are far more stable, and the instability of AsCl5 looks unusual. The cause is thought to be incomplete nucleus shielding in the 4p elements following the first transition series (i.e. gallium, germanium, arsenic, selenium, bromine, and krypton), which causes the 4s electrons to stabilize and become less available for bonding. This effect is known as the d-block contraction, and it is related to the f-block contraction, also known as the lanthanide contraction.
Uses
Arsenic pentachloride has limited practical applications. It is primarily used as a reagent in organic synthesis, particularly in the preparation of various organoarsenic compounds.
Toxicity
Arsenic compounds are generally toxic, and arsenic pentachloride is no exception. It is hazardous and should be handled with care in a controlled laboratory environment.