Catalysts are substances that speed up chemical reactions without being consumed in the process. In recent years, researchers have been exploring how catalysts can be used to purify contaminated water and produce hydrogen gas, a clean-burning fuel that could help to reduce greenhouse gas emissions.
Oregon State University College of Science researchers have developed a dual-purpose catalyst that purifies herbicide-contaminated water while also producing hydrogen. The project, which included researchers from the Ohio State University College of Engineering and HP Inc., is significant because water pollution is a major global issue, and hydrogen is a clean, renewable fuel.
The study’s findings, which looked into photoactive catalysts, were published today in the journal ACS Catalysis. “We can combine oxidation and reduction into a single process to achieve an efficient photocatalytic system,” said Kyriakos Stylianou of Ohio State University. “Oxidation happens via a photodegradation reaction, and reduction through a hydrogen evolution reaction.”
A catalyst is a substance that speeds up a chemical reaction without undergoing any permanent chemical changes. Photocatalysts are materials that absorb light to increase their energy level and then use that energy to oxidize organic contaminants. Self-cleaning coatings for stain- and odor-resistant walls, floors, ceilings, and furniture are among the many applications for photocatalysts.
We can combine oxidation and reduction into a single process to achieve an efficient photocatalytic system. Oxidation happens via a photodegradation reaction, and reduction through a hydrogen evolution reaction.
Kyriakos Stylianou
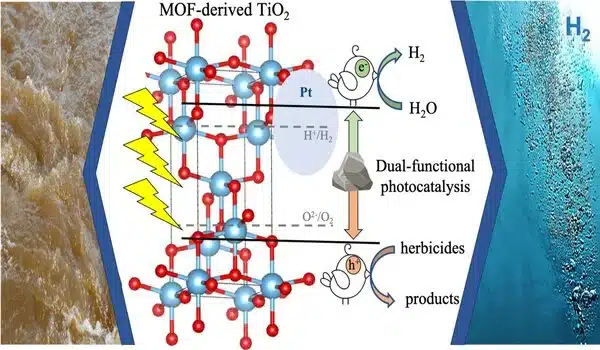
The research was led by Stylianou, an assistant professor of chemistry, and involved titanium dioxide photocatalysts derived from a metal-organic framework, or MOF.
MOFs are crystalline, porous materials with tunable structural properties and nanosized pores made up of positively charged metal ions surrounded by organic “linker” molecules. They can be built with a variety of components that determine the properties of the MOF.
Semiconducting materials such as titanium dioxide can be produced by calcining MOFs (high heating without melting). Titanium dioxide, found in the minerals anatase, rutile, and brookite, is the most commonly used photocatalyst.
Stylianou and colleagues, including Lney rnadóttir of the Ohio State University College of Engineering and William Stickle of HP, discovered that anatase doped with nitrogen and sulfur was the best “two birds, one stone” photocatalyst for producing hydrogen while also degrading the widely used herbicide glyphosate.
Glyphosate, also known as N-phosphonomethyl glycine or PMG, has been widely sprayed on agricultural fields over the last 50 years since its introduction under the brand name Roundup.
“Only a small portion of the total amount of PMG applied is taken up by crops, and the rest ends up in the environment,” Stylianou explained. “This raises concerns about the leaching of PMG into soil and groundwater, which is understandable given that contaminated water can be harmful to the health of all living things on the planet. Herbicides leaching into waterways are a major source of water pollution.”
Water is the most common compound in which hydrogen can be found, and producing hydrogen by splitting water via photocatalysis is cleaner and more sustainable than the traditional method of obtaining hydrogen – from natural gas through a carbon-dioxide-producing process known as methane-steam reforming.
Hydrogen serves many scientific and industrial purposes in addition to its energy-related roles. It’s used in fuel cells for cars, in the manufacture of many chemicals including ammonia, in the refining of metals and in the production of plastics.
“Water is a rich source of hydrogen, and photocatalysis is a method of harnessing the Earth’s abundant solar energy for hydrogen production and environmental remediation,” Stylianou explained. “We demonstrate that it is possible to produce a renewable fuel while removing organic pollutants or converting them into useful products using photocatalysis.”
The team, which included graduate student Emmanuel Musa, postdoctoral researcher Sumandeep Kaur, and students Trenton Gallagher and Thao Mi Anthony, also tested the photocatalyst against water contaminated with two other commonly used herbicides, glufosinate ammonium and 2,4-dichlorophenoxyacetic acid. It also worked on water containing them – even water containing those two compounds plus PMG.