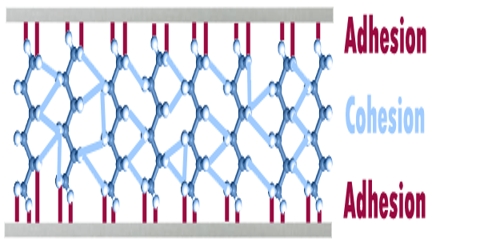
Adhesion and Cohesion
Attractive force operating between like molecules, as in a pure liquid, is termed the force of cohesion. The term adhesion is used if the attraction is between unlike molecules, namely, between water and a clean metal surface. When adhesion is relatively strong the water molecules will spread on the clean metal surface. This is known as wetting. The forces have decisive influence in choosing a correct detergent for cleaning purposes.
Cohesive forces are responsible for surface tension, the tendency of a liquid’s surface to resist rupture when placed under tension or stress.
Adhesion
- Adhesion is the force of attraction between two dissimilar molecules or substances.
- A best example for adhesion is the force of attraction lying between the walls of xylem vessels and the water molecules.
- Adhesion is the result of electrostatic or mechanical forces between two kinds of different substances.
- A stronger force of adhesion tends the liquid to spread over the surface
- Adhesion is the property of different molecules or surfaces to cling to each other.
- Effects: Capillary action, meniscus etc.
Cohesion
- Cohesion is the force of attraction between two similar substances or molecules.
- An example for the cohesion forces is the force of attraction between the water molecules.
- Cohesion is caused mainly due to the Van der Waals forces and hydrogen bonding.
- A strong force of cohesion tends to form water droplets on the surface.
- Cohesion is the property of like molecules to stick to each other due to mutual attraction.
- Effects: Surface tension, capillary action and meniscus etc.