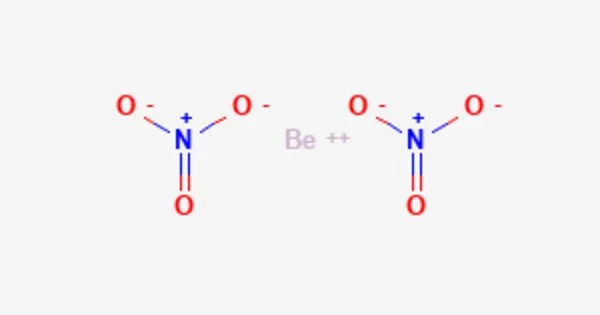
Beryllium nitrate, with the idealized chemical formula Be(NO3)2, is an inorganic compound. The formula suggests a salt, but the compound is highly covalent, as are many beryllium compounds. It is a crystalline solid that ranges from white to pale yellow. It dissolves in water. Little is known about its chemistry. Brown fumes are produced when added to water; when hydrolyzed in sodium hydroxide solution, both nitrate and nitrite ions are produced.
It is incombustible, but it will hasten the combustion of combustible materials. An explosion may occur if large quantities of the material are involved or the material is finely divided. An explosion may occur if exposed to fire or heat for an extended period of time. In fires involving this material, toxic oxides of nitrogen are produced. It is used in chemical analysis.
Properties
Beryllium nitrate is an ionic beryllium salt of nitric acid with the chemical formula Be(NO).Each formula unit is composed of one Be2+ cation and two NO-anions.
- Chemical formula: Be(NO3)2
- Molar mass: 133.021982 g/mol
- Appearance: white solid
- Odor: odorless
- Density: 1.56 g/cm3
- Melting point: 60.5 °C (140.9 °F; 333.6 K)
- Boiling point: 142 °C (288 °F; 415 K) (decomposes)
- Solubility in water: 166 g/100 mL

Synthesis and reactions
The straw-colored adduct Be(NO3)2(N2O4) forms upon treatment of beryllium chloride with dinitrogen tetroxide:
BeCl2 + 3 N2O4 → Be(NO3)2(N2O4) + 2 NOCl
Upon heating, this adduct loses N2O4 and produces colorless Be(NO3)2. Further heating of Be(NO3)2 induces conversion to basic beryllium nitrate, which adopts a structure akin to that for basic berylium acetate.
Unlike the basic acetate, with its six lipophilic methyl groups, the basic nitrate is insoluble in most solvents.
Preparation
Anhydrous beryllium nitrate was made by reacting anhydrous beryllium chloride with an ethylacetate-dinitrogen tetroxide mixture to produce Be(NO3)2. Oxides of nitrogen are formed when anhydrous beryllium nitrate is heated above 50 °C (120 °F) in a vacuum. The anhydrous salt is stable until about 125 °C (255 °F), at which point it decomposes into nitrogen tetroxide and a volatile compound that separates from the gas phase as colorless Be4O(NO6)6 crystals. When these crystals are immersed in water, they slowly hydrolyze.
Hazard
Beryllium nitrate is a toxic chemical,like all other beryllium compounds. It is also an irritant in small doses. When burned, it gives off irritating or toxic fumes. However, when massive short-term exposure occurs, acute pneumonitis can set in, but symptoms do not manifest themselves for 3 days.