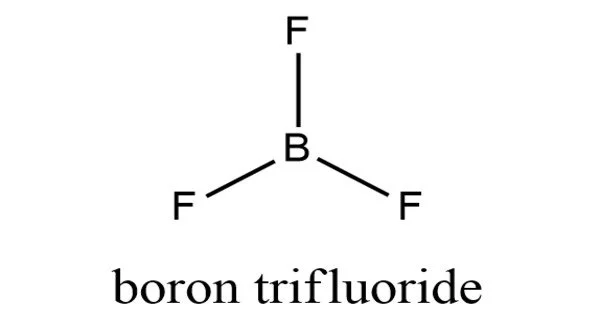
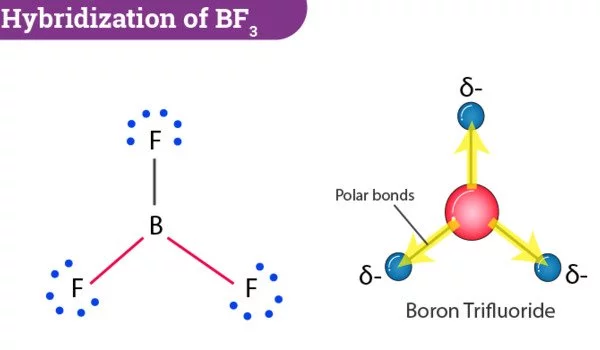
Boron trifluoride is an inorganic compound with the formula BF3. In moist air, this pungent, colorless, and toxic gas produces white fumes. It is a useful Lewis acid and a versatile building block for other boron compounds.
Boron trifluoride was discovered in 1808 by Joseph Louis Gay-Lussac and Louis Jacques Thénard, who were attempting to isolate “fluoric acid” (i.e., hydrofluoric acid) by combining calcium fluoride with vitrified boric acid. Because the resulting vapours failed to etch glass, they were dubbed fluoboric gas.
Properties
With a boiling point of 100.3 °C and a critical temperature of 12.3 °C, anhydrous boron trifluoride can only be stored as a refrigerated liquid between those temperatures. Because a refrigeration system failure could cause pressures to rise to the critical pressure of 49.85 bar, storage or transport vessels should be designed to withstand internal pressure (4.985 MPa).
- Chemical formula: BF3
- Molar mass: 67.82 g/mol (anhydrous); 103.837 g/mol (dihydrate)
- Appearance: colorless gas (anhydrous); colorless liquid (dihydrate)
- Density: 0.00276 g/cm3 (anhydrous gas); 1.64 g/cm3 (dihydrate)
- Melting point: −126.8 °C (−196.2 °F; 146.3 K)
- Boiling point: −100.3 °C (−148.5 °F; 172.8 K)
- Solubility in water: exothermic decomposition (anhydrous); very soluble (dihydrate)
- Solubility: soluble in benzene, toluene, hexane, chloroform and methylene chloride
- Vapor pressure: >50 atm (20 °C)
Boron trifluoride is extremely corrosive. Metals suitable for boron trifluoride handling equipment include stainless steel, monel, and hastelloy. It corrodes steel, including stainless steel, in the presence of moisture. It combines with polyamides. Polytetrafluoroethylene, polychlorotrifluoroethylene, polyvinylidene fluoride, and polypropylene all exhibit adequate resistance. Because boron trifluoride reacts with hydrocarbon-based greases, the grease used in the equipment should be fluorocarbon-based.
Synthesis and handling
BF3 is manufactured by the reaction of boron oxides with hydrogen fluoride:
B2O3 + 6 HF → 2 BF3 + 3 H2O
Typically the HF is produced in situ from sulfuric acid and fluorite (CaF2). Approximately 2300-4500 tonnes of boron trifluoride are produced every year.
Laboratory scale
For laboratory scale reactions, BF3 is usually produced in situ using boron trifluoride etherate, which is a commercially available liquid.
Laboratory routes to the solvent-free materials are numerous. A well documented route involves the thermal decomposition of diazonium salts of BF4–:
PhN2BF4 → PhF + BF3 + N2
Alternatively it arises from the reaction of sodium tetrafluoroborate, boron trioxide, and sulfuric acid:
6 NaBF4 + B2O3 + 6 H2SO4 → 8 BF3 + 6 NaHSO4 + 3 H2O
Reactions
Unlike the aluminium and gallium trihalides, the boron trihalides are all monomeric. They undergo rapid halide exchange reactions:
BF3 + BCl3 → BF2Cl + BCl2F
Because of the facility of this exchange process, the mixed halides cannot be obtained in pure form.
Boron trifluoride is a versatile Lewis acid that forms adducts with such Lewis bases as fluoride and ethers:
CsF + BF3 → CsBF4
O(C2H5)2 + BF3 → BF3·O(C2H5)2
As non-coordinating anions, tetrafluoroborate salts are commonly used. The adduct with diethyl ether, boron trifluoride diethyl etherate, or simply boron trifluoride etherate, (BF3•O(Et)2), is a liquid that can be easily handled and is thus commonly used as a laboratory source of BF3. The adduct with dimethyl sulfide (BF3•S(Me)2) is another common adduct that can be handled as a neat liquid.