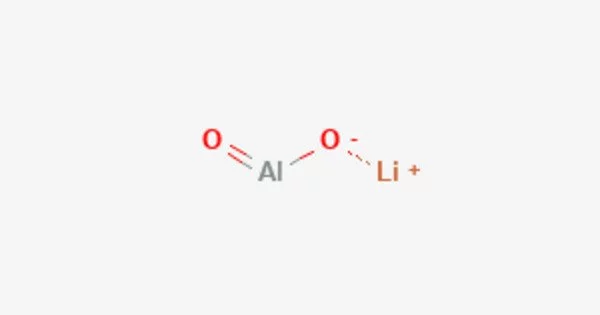
Lithium aluminate (LiAlO2) is an inorganic compound that consists of lithium, aluminum, and oxygen. It is a solid, white powder that is insoluble in water. It is a ceramic material that is commonly used as a substrate for the growth of thin films in the electronics industry.
Lithium aluminate has a crystal structure that belongs to the trigonal crystal system. It is a colorless or white crystalline powder that is insoluble in water but soluble in acid. It is typically synthesized by the reaction of lithium carbonate or lithium hydroxide with aluminum hydroxide or aluminum oxide at high temperatures.
Properties
- Physical Properties: It is a white crystalline powder that is insoluble in water. It has a melting point of 1892°C and a density of 2.6 g/cm³.
- Chemical Properties: It is a stable compound that does not react with water or acids. It is a good electrical insulator and has a high dielectric constant.
- Thermal Properties: It has a high thermal conductivity and a low thermal expansion coefficient, making it a useful material in high-temperature applications.
- Mechanical Properties: It has a high strength and hardness, and it is resistant to wear and abrasion. It also has good fracture toughness and can withstand high stresses.
- Optical Properties: It is transparent to visible and near-infrared light and has a high refractive index. It is often used as a substrate for optical coatings and filters.
Overall, lithium aluminate is a versatile material with a range of useful properties that make it suitable for a variety of applications, including in electronics, ceramics, and optics.
Application
Lithium aluminate has several applications, including as a catalyst support, as a material for gas sensors, as a substrate for epitaxial growth of thin films, and as a component in solid-state electrolytes for lithium-ion batteries.
It is also used in the production of various high-performance materials such as ceramics, glasses, and refractory materials due to its excellent thermal stability, high melting point, and good electrical conductivity.