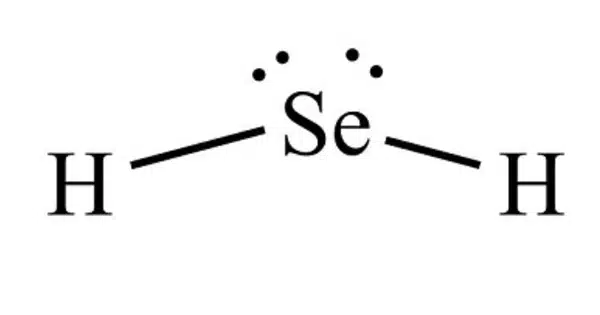
Hydrogen selenide is an inorganic compound with the formula H2Se. This hydrogen chalcogenide is the most basic and widely encountered selenium hydride. Under normal circumstances, H2Se is a colourless, flammable gas. It is the most toxic selenium compound, with an exposure limit of 0.05 ppm over an 8-hour period.
Even at extremely low concentrations, this compound has a very unpleasant odour that resembles decayed horseradish or ‘leaking gas,’ but at higher concentrations, it smells like rotten eggs. It is a colourless gas with a strong odour. It is highly toxic and can be harmful to human health, so it should be handled with caution.
Properties
- Chemical formula: H2Se
- Molar mass: 80.98 g/mol
- Appearance: Colorless gas
- Odor: decayed horseradish
- Density: 3.553 g/dm3
- Melting point: −65.73 °C (−86.31 °F; 207.42 K)
- Boiling point: −41.25 °C (−42.25 °F; 231.90 K)
- Solubility in water: 0.70 g/100 mL
- Solubility: soluble in CS2, phosgene
- Vapor pressure: 9.5 atm (21°C)
- Conjugate acid: Selenonium
- Conjugate base: Selenide
Structure and properties
H2Se adopts a bent structure with a H−Se−H bond angle of 91°[citation needed]. Consistent with this structure, three IR-active vibrational bands are observed: 2358, 2345, and 1034 cm−1.
The properties of H2S and H2Se are similar, although the selenide is more acidic with pKa = 3.89 and the second pKa = 11, or 15.05 ± 0.02 at 25 °C.
Preparation
It is manufactured industrially by treating elemental selenium with hydrogen gas at temperatures above T > 300 °C. There have been several reported routes to H2Se that are suitable for both large and small scale preparations. In the laboratory, H2Se is typically produced by the action of water on Al2Se3, which also results in the formation of hydrated alumina. The acid hydrolysis of FeSe is a related reaction.
Al2Se3 + 6 H2O ⇌ 2 Al(OH)3 + 3 H2Se
H2Se can also be produced in situ in aqueous solution using boron hydride, the Marsh test, and Devarda’s alloy, among other methods. The Sonoda method generates H2Se from the reaction of H2O and CO on Se in the presence of Et3N. H2Se is available in cylinders.
Reactions
Elemental selenium can be recovered from H2Se through a reaction with aqueous sulfur dioxide (SO2).
2 H2Se + SO2 ⇌ 2 H2O + 2 Se + S
Its decomposition is used to prepare the highly pure element.
Application
The primary application of hydrogen selenide is in chemical research and synthesis. It is created by combining hydrogen gas (H2) and elemental selenium (Se). However, because of its toxicity and danger, its applications are limited.
Health hazards
When hydrogen selenide gas is released into the atmosphere, it can combine with oxygen to form selenium dioxide (SeO2), a toxic and volatile compound. Inhaling hydrogen selenide gas can cause respiratory and eye irritation, as well as other health issues. As a result, it is critical to handle and store hydrogen selenide safely, such as in a well-ventilated area and with protective equipment.