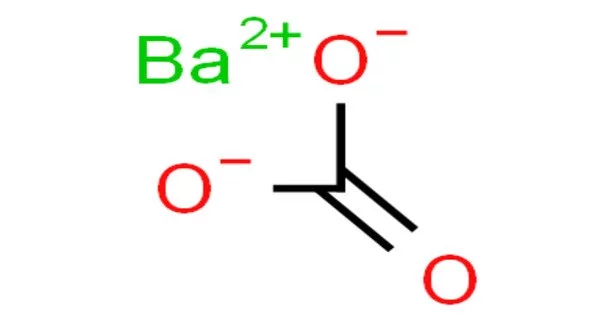
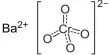
The inorganic compound with the formula BaCO3 is barium carbonate. It looks like white powder. It is a white salt that is poorly soluble in water, like most alkaline earth metal carbonates. With the exception of sulfuric acid, it is insoluble in water and soluble in most acids. It is found as the mineral witherite. The specific gravity is 4.275. It is one of the most important barium compounds in terms of commerce. It is toxic when consumed.
Many major commercial applications for barium carbonate exist in the glass, brick, oil-drilling, ceramics, photographic, and chemical industries. It is used as a rodenticide and has a similar appearance to flour, which has been responsible for the majority of unintentional barium poisoning.
Properties
Barium carbonate is a white, odorless, slightly soluble in water, slightly soluble in ethanol; it is a basic carbonate of barium; it is used in the manufacture of glass, ceramics, and other inorganic chemicals.
- Chemical formula: BaCO3
- Molar mass: 197.34 g/mol
- Appearance: white crystals
- Odor: odorless
- Density: 4.286 g/cm3
- Melting point: 811 °C (1,492 °F; 1,084 K); polymorphic transformation
- Boiling point: 1,450 °C (2,640 °F; 1,720 K); decomposes from 1360 °C
- Solubility in water: 16 mg/L (8.8°C); 22 mg/L (18 °C); 24 mg/L (20 °C)
- Solubility product (Ksp): 2.58·10−9
- Solubility: decomposes in acid; insoluble in methanol

Preparation
Barium carbonate is made commercially from barium sulfide by treatment with sodium carbonate at 60 to 70 °C (soda ash method) or, more commonly carbon dioxide at 40 to 90 °C:
In the soda ash process, an aqueous solution of barium sulfide is treated with sodium carbonate:
BaS + H2O + CO2 → BaCO3 + H2S
Precipitation can also be used to prepare it from barytes. It can also be seen in turquoise glazes. It is recommended that you use proper protective measures when working with this chemical compound because of its high toxicity. It should be strictly limited to low quality, preferably less than 20%. Barium Monocarbonate is another name for it.
Reactions
Barium carbonate reacts with acids such as hydrochloric acid to form soluble barium salts, such as barium chloride:
BaCO + 2 HCl → BaCl2 + CO2 + H2O
Pyrolysis of barium carbonate gives barium oxide.
Uses
Barium carbonate is a white insoluble salt which finds its largest use in the ceramics industry in the production of ceramic products. It is mainly used to remove sulfate impurities from feedstock of the chlor-alkali process. Otherwise, it is a common precursor to barium-containing compounds such as ferrites.
Other uses
Barium carbonate is a common ingredient in glazes in the ceramics industry. It acts as a flux, a matting and crystallizing agent, and it combines with specific coloring oxides to produce unique colors that are difficult to achieve through other means. Its use is somewhat contentious because some claim that it can leach from glazes into food and beverages. BaO is frequently used in fritted form to provide a safe means of use.
Barium carbonate is added to clays in the brick, tile, earthenware, and pottery industries to precipitate soluble salts (calcium sulfate and magnesium sulfate) that cause efflorescence.