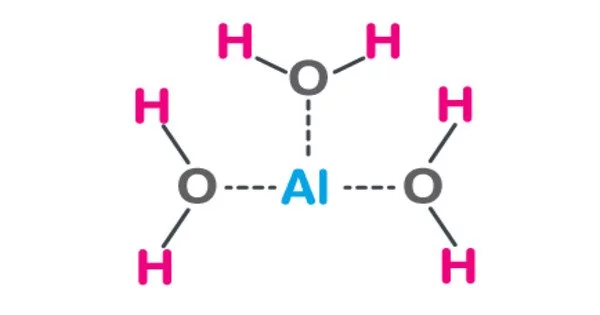
Aluminium hydroxide is a chemical compound with the chemical formula Al(OH)3. The mineral gibbsite (also known as hydrargillite) and its three much rarer polymorphs, bayerite, doyleite, and nordstrandite, are all found in nature as aluminium hydroxide, Al(OH)3. Aluminium hydroxide is amphoteric, which means it is both basic and acidic.
Aluminium oxide hydroxide, AlO(OH), and aluminum oxide or alumina (Al2O3), both of which are amphoteric, are closely related. These compounds are the primary constituents of the aluminum ore bauxite. In water, aluminium hydroxide also forms a gelatinous precipitate. It is an inorganic compound that is commonly used as an antacid to neutralize stomach acid in people with heartburn or indigestion.
Properties
Aluminium hydroxide is a white, odorless, and tasteless powder that is insoluble in water. It is formed by the reaction of aluminium salts with sodium or potassium hydroxide. When it is ingested, aluminium hydroxide reacts with the hydrochloric acid in the stomach to form aluminium chloride and water, which helps to neutralize excess stomach acid.
- Chemical formula: Al(OH)3
- Molar mass: 78.00 g/mol
- Appearance: White amorphous powder
- Density: 2.42 g/cm3, solid
- Melting point: 300 °C (572 °F; 573 K)
- Solubility in water: 0.0001 g/100 mL
- Solubility product (Ksp): 3×10−34
- Solubility: soluble in acids and alkalis
- Acidity (pKa): >7
- Isoelectric point: 7.7
Aluminium hydroxide is amphoteric. In acid, it acts as a Brønsted–Lowry base. It neutralizes the acid, yielding a salt:
3 HCl + Al(OH)3 → AlCl3 + 3 H2O
In bases, it acts as a Lewis acid by binding hydroxide ions:
Al(OH)3 + OH− → Al(OH)4−

Production
The Bayer process, which involves dissolving bauxite in sodium hydroxide at temperatures up to 270 °C (518 °F), produces nearly all of the aluminium hydroxide used commercially. Bauxite tailings are removed as waste, and aluminium hydroxide is precipitated from the remaining sodium aluminate solution. Calcination can convert this aluminium hydroxide to aluminum oxide or alumina.
Because of the residual sodium hydroxide, the residue or bauxite tailings, which is mostly iron oxide, is highly caustic. It was previously stored in lagoons, which resulted in the 2010 Ajka alumina plant accident in Hungary, in which a dam burst, drowning nine people. Another 122 people sought treatment for chemical burns. The mud contaminated 40 square kilometers (15 sq mi) of land and reached the Danube. While the mud was considered non-toxic due to low levels of heavy metals, the associated slurry had a pH of 13.
Application
Aluminium hydroxide is widely used as an antacid to neutralize excess stomach acid. It works by reacting with the hydrochloric acid in the stomach to form aluminum chloride and water, which helps to reduce the acidity of the stomach. It is also used in the manufacturing of various other products such as paper, ceramics, and plastics.
In addition, aluminium hydroxide is used as a flame retardant in plastics, textiles, and other materials. When exposed to heat, it decomposes to release water, which cools the material and reduces the risk of combustion. It is also used in water treatment to remove impurities such as heavy metals, and in the production of certain pigments and dyes.
Safety hazards
In addition to its use as an antacid, aluminium hydroxide is also used in the production of aluminum metal, in the manufacture of ceramics and glass, and as a fire retardant in plastics and textiles. However, prolonged exposure to high levels of aluminium has been associated with health problems such as Alzheimer’s disease, so its use is regulated by various government agencies.