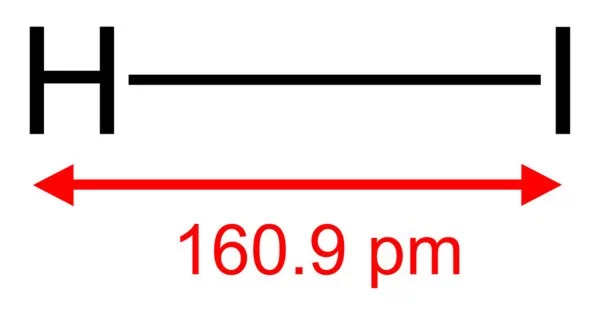
Hydrogen iodide (HI) is a hydrogen halide and a diatomic molecule. It is a chemical compound made up of the elements hydrogen (H) and iodine (I). At room temperature, it is a colourless gas that is highly soluble in water, forming a strong acid solution. Aqueous HI solutions are known as hydroiodic acid or hydriodic acid, both of which are strong acids.
However, hydrogen iodide and hydroiodic acid differ in that the former is a gas under normal conditions, whereas the latter is an aqueous solution of the gas. They are interchangeable. HI is used in organic and inorganic synthesis as a reducing agent and as a primary source of iodine.
Properties
HI is a colorless gas that reacts with oxygen to give water and iodine. With moist air, HI gives a mist (or fumes) of hydroiodic acid. It is exceptionally soluble in water, giving hydroiodic acid. One liter of water will dissolve 425 liters of HI gas, the most concentrated solution having only four water molecules per molecule of HI.
- Chemical formula: HI
- Molar mass: 127.904 g/mol
- Appearance: Colorless gas
- Odor: acrid
- Density: 2.85 g/mL (−47 °C)
- Melting point: −50.80 °C (−59.44 °F; 222.35 K)
- Boiling point: −35.36 °C (−31.65 °F; 237.79 K)
- Solubility in water: approximately 245 g/100 ml
- Conjugate acid: Iodonium
- Conjugate base: Iodide
Hydrogen iodide exists as a gas at room temperature and atmospheric pressure. However, it readily dissolves in water, forming a solution of hydroiodic acid. It has a pungent and irritating odor. It is highly soluble in water, and the resulting solution is a strong acid. HI is a strong reducing agent and reacts readily with many substances, including metals and oxidizing agents.
Preparation
Hydrogen iodide can be prepared by the direct combination of hydrogen gas (H2) and iodine gas (I2). The reaction is exothermic and typically occurs in the presence of a catalyst, such as phosphorus, iron, or red phosphorus.
Uses
- HI is commonly used in various chemical reactions as a source of iodine or as a reducing agent.
- It is employed in organic synthesis, particularly for the preparation of iodinated organic compounds.
- It is used as a reducing agent and in analytical procedures for the determination of certain elements.
- HI is utilized in the production of certain pharmaceuticals, particularly those containing iodine.
Safety Considerations
Hydrogen iodide is a corrosive and toxic substance. It is hazardous if inhaled, ingested, or if it comes into contact with the skin or eyes. Proper precautions, such as the use of protective equipment and working in a well-ventilated area, should be taken when handling this compound.