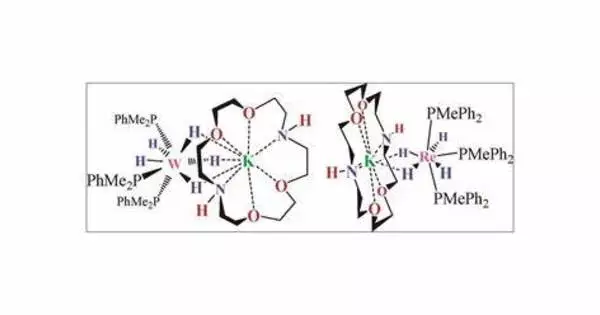
A polyhydride or superhydride is a compound with an unusually high hydrogen content. These are compounds containing multiple hydrogen atoms, which are frequently combined with other elements. This is known as high hydrogen stoichiometry. Iron pentahydride FeH5, LiH6, and LiH7 are a few examples. In comparison, the more well-known lithium hydride contains only one hydrogen atom. Only under extreme pressure are these known to be stable.
The methods for synthesizing polyhydrides differ depending on the compound. Some can be made via direct reactions, while others may necessitate more complex synthesis routes.
Properties
The properties of polyhydrides can vary widely depending on the specific elements involved, their chemical structure, and the conditions under which they exist.
- Chemical Reactivity: Because of the presence of multiple hydrogen atoms, which are prone to participating in chemical reactions, these can be highly reactive. The reactivity of the compound may be affected by the other elements present. Metal polyhydrides, for example, may be more reactive than non-metal polyhydrides.
- Stability: Polyhydrides’ stability varies. Some polyhydrides are stable under normal conditions, while others may be heat, light, or air sensitive. Certain metal polyhydrides may be highly stable, whereas hydrides of lighter nonmetals may be less stable.
- Physical State: These can exist in different physical states, such as gases, liquids, or solids, depending on the specific compound and its conditions.
- Density: The density can vary widely. Metal hydrides, for example, are often dense materials.
- Hydrogen Storage: These are often studied for their potential in hydrogen storage. Some compounds can absorb and release hydrogen, which is of interest in the development of hydrogen storage systems for fuel cells and other applications.
Applications
Polyhydrides, depending on their properties, may find use in a variety of fields, including catalysis, hydrogen storage, and materials science.
Polyhydrides are significant because they can form substances with extremely high hydrogen densities. They resemble the elusive metallic hydrogen, but they can be produced at lower pressures. One theory is that they are superconductors. At 203 K (70 °C) and 1.5 million atmospheres of pressure, hydrogen sulfide forms SH3 units and can be a superconductor.