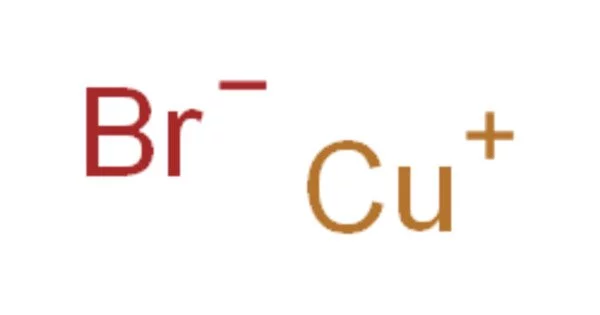The chemical compound with the formula CuBr is copper(I) bromide. This diamagnetic solid has a polymeric structure similar to zinc sulfide. Copper bromide is the name given to the man-made ionic compound formed by the fusion of copper and bromine. The compound is widely used in organic compound synthesis and as a lasing medium in copper bromide lasers. Because it is formed by the interaction of metal copper and nonmetal bromine, it is an ionic compound.
Properties
It is naturally white but may appear-grayish black due to impurities present in copper. It is soluble in solvents such as alcohol, acetone, and ammonia and insoluble in solvents such as benzene, sulfuric acid, ethyl ether, etc. The molar mass of the compound is 223.37 g/mol. Its melting point and boiling point are 928.4°F and 1652°F respectively.
- Chemical formula: CuBr
- Molar mass: 143.45 g/mol
- Appearance: white powder (see text)
- Density: 4.71 g/cm3, solid
- Melting point: 492 °C (918 °F; 765 K)
- Boiling point: 1,345 °C (2,453 °F; 1,618 K)
- Solubility in water: slightly soluble
- Solubility product (Ksp): 6.27×10-9
- Solubility: soluble in HCl, HBr, ammonium hydroxide, acetonitrile, negligible in acetone, sulfuric acid
- Dipole moment: 1.46 D
- Flash point: Non-flammable

Structure
Although the compound is white, samples are frequently colored due to the presence of copper(II) impurities. In addition, the copper(I) ion oxidizes readily in air. It is typically made by reducing cupric salts with sulfite in the presence of bromide. For example, copper(II) bromide reduced with sulfite yields copper(I) bromide and hydrogen bromide:
2 CuBr2 + H2O + SO32- → 2 CuBr + SO42- + 2 HBr
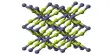
CuBr is insoluble in most solvents due to its polymeric structure, which features four-coordinated, tetrahedral Cu centers interconnected by bromide ligands (ZnS structure). Upon treatment with Lewis bases, CuBr converts to molecular adducts. For example, with dimethyl sulfide, the colorless complex is formed:
CuBr + S(CH3)2 → CuBr(S(CH3)2)
The copper in this coordination complex is two-coordinate and has a linear geometry. Other soft ligands provide similar complexes. Triphenylphosphine, for example, gives CuBr(P(C6H5)3), though this species has a more complex structure. Thermal excitation of copper(I) bromide vapour results in a more saturated blue-violet emission than known copper(I) chloride emission. As a result, copper(I) bromide is an advantageous emitter in pyrotechnic flames.
Applications in organic chemistry
In the Sandmeyer reaction, CuBr is employed to convert diazonium salts into the corresponding aryl bromides:
ArN+2 + CuBr → ArBr + N2 + Cu+
The aforementioned complex CuBr(S(CH3)2) is widely used to generate organocopper reagents. Related CuBr complexes are catalysts for atom transfer radical polymerization and copper-catalyzed cross-dehydrogenative couplings (CDC).